Abstract
A one-pot method was introduced to fabricate Ag–SiO2 hollow composite spheres with developed porosity by employing monodisperse carboxylic-capped polystyrene (PS–COOH) spheres as a hard template. During the synthetic process, Ag+ ions were immediately anchored on the surface of the PS–COOH spheres via electrostatic interaction and readily reduced by employing polymethylhydrosiloxane (PMHS) as a reducing agent. At the same time, silica oligomers derived from the hydrolysis of PMHS and TEOS were slowly deposited on the PS–COOH spheres to form solid silica shells. Subsequent decomposition of the PS–COOH spheres in air induced the formation of Ag–SiO2 hollow spheres. The as-prepared products were characterized by X-ray diffraction, Fourier transform infrared spectroscopy, scanning electron microscopy, transmission electron microscopy and N2 absorption–desorption study. The results show that the well-designed composite spheres featured thin and spherical silica shell (~10 nm), large surface area (>240 m2 g−1) and mesopore (6.0–9.0 nm). In addition, the Ag–SiO2 hollow composite spheres exhibited high catalytic performance toward the reduction of 4-nitrophenol (4-NP) to 4-aminophenol (4-AP).
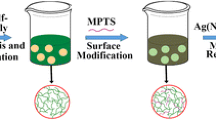
Graphical Abstract
A one-pot method was reported to prepare Ag–SiO2 hollow spheres with well-developed porosity, which exhibited high catalytic performance and durable activity for the reaction of 4-NP to 4-AP.








Similar content being viewed by others
References
Simonato S, Groger H, Mollmer J, Staudt R, Puls A, Dreisbach F, Feldmann C (2012) Sorption and separation of CO2 via nanoscale AlO(OH) hollow spheres. Chem Commun 48:844
Zhang J, Li B, Yang W (2014) Synthesis and photocatalytic performance of novel hierarchical hollow silica sphere supported TiO2 nanoparticles. Mater Lett 117:252
Cheng X, Li J, Li X, Zhang D, Zhang H, Zhang A, Huang H, Lian J (2012) A highly sensitive sensor based on hollow particles for the detection, adsorption and removal of Hg2+ ions. J Mater Chem 22:24102
Kraskiewicz H, Breen B, Sargeant T, McMahon S, Pandit A (2013) Assembly of protein-based hollow spheres encapsulating a therapeutic factor. ACS Chem Neurosci 4:1297
Yu M, Wang H, Zhou X, Yuan YuC (2007) One template synthesis of raspberry-like hierarchical siliceous hollow spheres. J Am Chem Soc 129:14576
Davis SA, Burkett SL, Mendelson NH, Mann S (1997) Bacterial templating of ordered macrostructures in silica and silica-surfactant mesophases. Nature 385:420
Shiomi T, Tsunoda T, Kawai A, Mizukami F, Sakaguchi K (2007) Formation of cage-like hollow spherical silica via a mesoporous structure by calcination of lysozyme–silica hybrid particles. Chem Commun 4404
Wang J, Xiao Q, Zhou H, Sun P, Yuan Z, Li B, Ding D, Shi A, Chen T (2006) Budded, mesoporous silica hollow spheres: hierarchical structure controlled by kinetic self-assembly. Adv Mater 18:3284
Wang JG, Li F, Zhou HJ, Sun PC, Ding DT, Chen TH (2009) Silica hollow spheres with ordered and radially oriented amino-functionalized mesochannels. Chem Mater 21:612
Fang X, Zang J, Wang X, Zheng MS, Zheng N (2014) A multiple coating route to hollow carbon spheres with foam-like shells and their applications in supercapacitor and confined catalysis. J Mater Chem A 2:6191
Hu J, Chen M, Fang X, Wu L (2011) Fabrication and application of inorganic hollow spheres. Chem Soc Rev 40:5472
Shi S, Wang M, Chen C, Gao J, Ma H, Ma J, Xu J (2013) Super-hydrophobic yolk–shell nanostructure with enhanced catalytic performance in the reduction of hydrophobic nitroaromatic compounds. Chem Commun 49:9591
Yin Y, Chen M, Zhou S, Wu L (2012) A general and feasible method for the fabrication of functional nanoparticles in mesoporous silica hollow composite spheres. J Mater Chem 22:11245
Zhang H, Huang H, Liu Y, Han X, Ma Z, Zhang L, Li H, Kang Z (2012) Porous and hollow metal-layer@SiO2 nanocomposites as stable nanoreactors for hydrocarbon selective oxidation. J Mater Chem 22:20182
Sora GD, Dalcanale F, Campostrini R, Gaston A, Blum Y, Carturanc S, Aravindxa PR (2012) Novel polysiloxane and polycarbosilane aerogels via hydrosilylation of preceramic polymers. J Mater Chem 22:7676
Zhai SR, Shao X, Zhou D, Zhai B, An QD (2012) Sol-gel synthesis of nanosilver embedded hybrid materials using combined organosilica precursors. J Sol-Gel Sci Technol 62:281
Acknowledgments
Financial supports from the Educational Commission of Liaoning Province of China (number L2013218), the Science and Technology Program of Dalian (2013A16GX117) and the Program for Liaoning Innovative Research Team in University (LT2013012) are highly appreciated.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Xiao, ZY., Huang, SX., Zhai, SR. et al. PMHS-reduced fabrication of hollow Ag–SiO2 composite spheres with developed porosity. J Sol-Gel Sci Technol 75, 82–89 (2015). https://doi.org/10.1007/s10971-015-3679-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-015-3679-3