Abstract
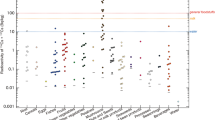
We analyzed 134Cs, 137Cs and 40K in 96 foodstuffs in supermarkets with high sensitivity over 3 years after Fukushima accident. Milk, yoghurt, rice, tea, salmon, cereal, blueberry, miso, and apples had a trace of 134Cs and 137Cs from 10−3 to 100 Bq/kg, however, some mushrooms that were bought in the outer Fukushima prefecture were contaminated by radioactive cesium over the regulatory limit (100 Bq/kg). In view of the 134Cs/137Cs radioactivity ratio, we can conclude that 137Cs detected in remote areas 300 km or more from Fukushima Nuclear power plant contained activity from Pre-Fukushima events such as Chernobyl accident (1986) and atmospheric nuclear explosions (from 1945).




Similar content being viewed by others
References
Steinhauser G, Brandl A, Johnson TE (2014) Comparison of the Chernobyl and Fukushima nuclear accidents: a review of the environmental impacts. Sci Total Environ 470–471:800–817. doi:10.1016/j.scitotenv.2013.10.029
Tagami K, Uchida S, Ishii N, Zheng J (2013) Estimation of Te-132 distribution in Fukushima Prefecture at the early stage of the Fukushima Daiichi Nuclear Power Plant reactor failures. Environ Sci Technol 47(10):5007–5012. doi:10.1021/es304730b
Chino M, Nakayama H, Nagai H, Terada H, Katata G, Yamazawa H (2011) Preliminary estimation of release amounts of 131I and137Cs Accidentally Discharged from the Fukushima Daiichi Nuclear Power Plant into the atmosphere. J Nucl Sci Technol 48(7):1129–1134. doi:10.1080/18811248.2011.9711799
Nishihara K, Yamagishi I, Yasuda K, Ishimori K, Tanaka K, Kuno T, Inada S, Gotoh Y (2012) Radionuclide release to stagnant water in Fukushima-1 Nuclear Power Plant. Trans Atomic Energ Soc Jpn 11(1):13–19. doi:10.3327/taesj.J11.040
Tsumune D, Tsubono T, Aoyama M, Hirose K (2012) Distribution of oceanic 137Cs from the Fukushima Dai-ichi Nuclear Power Plant simulated numerically by a regional ocean model. J Environ Radioactiv 111:100–108. doi:10.1016/j.jenvrad.2011.10.007
Winiarek V, Bocquet M, Saunier O, Mathieu A (2012) Estimation of errors in the inverse modeling of accidental release of atmospheric pollutant: application to the reconstruction of the cesium-137 and iodine-131 source terms from the Fukushima Daiichi power plant. J Geophys Res. doi:10.1029/2011jd016932
Stohl A, Seibert P, Wotawa G, Arnold D, Burkhart JF, Eckhardt S, Tapia C, Vargas A, Yasunari TJ (2012) Xenon-133 and caesium-137 releases into the atmosphere from the Fukushima Dai-ichi nuclear power plant: determination of the source term, atmospheric dispersion, and deposition. Atmos Chem Phys 12(5):2313–2343. doi:10.5194/acp-12-2313-2012
Morino Y, Ohara T, Watanabe M, Hayashi S, Nishizawa M (2013) Episode analysis of deposition of radiocesium from the Fukushima Daiichi nuclear power plant accident. Environ Sci Technol 47(5):2314–2322. doi:10.1021/es304620x
Teppei J, Yasunaria AS, Hayano Ryugo S, Burkhartb John F, Eckhardtb Sabine, Yasunarie Tetsuzo (2011) Cesium-137 deposition and contamination of Japanese soils due to the Fukushima nuclear accident. Proc Nat Acad Sci. doi:10.1073/pnas.1112058108/-/DCSupplemental
Fukushima Association for Securing Safety of Agricultural Products. https://fukumegu.org/ok/kome/
Shiraishi K, Ban-Nai T, Muramatsu Y, Yamamoto M (1999) Comparison of stable cesium and radiocesium on dietary intakes by Japanese subjects using 18 food categories. J Radioanal Nucl Chem 242(3):687–692. doi:10.1007/Bf02347380
Ministry of Health LaW, Japan (2013) National Health and Nutrition Survey
Becker W (2000) Total Diet Studies Examples from Sweden. J Food Compos Anal 13:545–549. doi:10.1006/jfca.2000.0907
Ministry of Education C, Sports, Science and Technology (MEXT), Japan (1982) The guideline of sample pre-treatment for Ge semiconductor defector. vol 14 (in Japanese)
ICRP (1995) Age-dependent doses to the members of the public from intake of radionuclides—part 5 compilation of ingestion and inhalation coefficients (Publication 72). vol 26
Ministry of Health LaW, Japan (2014) The results of surveys on the levels of radioactive cesium in foods (February–March 2014); 1% or below the current regulatory dose limit of 1 millisieverts per year (in Japanese)
Merz S, Shozugawa K, Steinhauser G (2015) Analysis of Japanese radionuclide monitoring data of food before and after the fukushima nuclear accident. Environ Sci Technol 49(5):2875–2885. doi:10.1021/es5057648
Guillen J, Baeza A (2014) Radioactivity in mushrooms: a health hazard? Food Chem 154:14–25. doi:10.1016/j.foodchem.2013.12.083
Lin C, Skarpelos J (1997) Monitoring of fission product release in a boiling water reactor. J Radioanal Nucl Chem 220(2):173–181. doi:10.1007/BF02034852
Merz S, Steinhauser G, Hamada N (2013) Anthropogenic radionuclides in Japanese food: environmental and legal implications. Environ Sci Technol 47(3):1248–1256. doi:10.1021/es3037498
Acknowledgments
Dr. Akira Ishikawa is acknowledged for providing access to the ICP-MS. This work was supported by JSPS KAKENHI Grant Number 25870158.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shozugawa, K., Saito, T., Hori, M. et al. High-sensitivity determination of radioactive cesium in Japanese foodstuffs: 3 years after the Fukushima accident. J Radioanal Nucl Chem 307, 2117–2122 (2016). https://doi.org/10.1007/s10967-015-4407-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-015-4407-8