Abstract
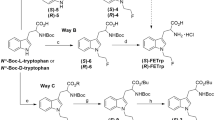
Due to favourable in vivo characteristics, its high specificity and the longer half-life of 18F (109.8 min) allowing for remote-site delivery, O-(2-[18F]fluoroethyl)-l-tyrosine ([18F]FET) has gained increased importance for molecular imaging of cerebral tumors. Consequently, the development of simple and efficient production strategies for [18F]FET could be an important step to further improve the cost-effective availability of [18F]FET in the clinical environment. In the present study [18F]FET was synthesized via direct nucleophilic synthesis using an earlier developed chiral precursor, the NiII complex of an alkylated (S)-tyrosine Schiff base, Ni-(S)-BPB-(S)-Tyr-OCH2CH2OTs. The purification method has been developed via solid phase extraction thereby omitting cumbersome HPLC purification. The suggested SPE purification using combination of reverse phase and strong cation exchange cartridges provided [18F]FET in high chemical, radiochemical and enantiomeric purity and 35 % radiochemical yield (decay-corrected, 45 min synthesis time). The method was successfully automated using a commercially available synthesis module, Scintomics Hotboxone. Based on the current results, the proposed production route appears to be well suited for transfer into an automated cassette-type radiosynthesizers without using HPLC.




Similar content being viewed by others
References
Coenen HH, Elsinga PH, Iwata R, Kilbourn MR, Pillai MRA, Rajan MGR, Wagner HN, Zaknun JJ (2010) Fluorine-18 radiopharmaceuticals beyond [18F]FDG for use in oncology and neurosciences. Nucl Med Biol 37:727–740
Wester H, Herz M, Weber W, Heiss P, Senekovitsch-Schmidtke R, Schwaiger M, Stocklin G (1999) Synthesis and radiofarmacology of O-(2-[18F] fluoroethyl)-l-tyrosine for tumor imaging. J Nucl Med 40:205–212
Popperl G, Kreth FW, Mehrkens JH, Herms J, Seelos K, Koch W, Gildehaus FJ, Kretzschmar HA, Tonn JC, Tatsch K (2007) FET PET for the evaluation of untreated gliomas: correlation of FET uptake and uptake kinetics with tumour grading. Eur J Nucl Med Mol Imaging 34:1933–1942
Galldiks N, Stoffels G, Filss CP, Piroth MD, Sabel M, Ruge MI, Herzog H, Shah NJ, Fink GR, Coenen HH, Langen KJ (2012) Role of O-(2-18F-fluoroethyl)-l-tyrosine PET for differentiation of local recurrent brain metastasis from radiation necrosis. J Nucl Med 53:1367–1374
Dunet V, Rossier C, Buck A, Stupp R, Prior JO (2012) Performance of 18F-fluoro-ethyl-tyrosine (18F-FET) PET for the differential diagnosis of primary brain tumor: a systematic review and meta analysis. J Nucl Med 53:207–214
Mueller D, Klette I, Kalb F, Baum RP (2011) Synthesis of O-(2′-[18F]fluoroethyl)-l-tyrosine based on a cartridge purification method. Nucl Med Biol 38(5):653–658
Gomzina NA, Vassiliev DA, Krasikova RN (2007) The application of 2-[18F]fluoroethylbromide in the synthesis of O-(2′-[18F]fluoroethyl)-l-tyrosine, radiopharmaceutical for brain tumors diagnosis by PET. Radiochemistry 49(3):264–269
Zuhayra M, Alfteimi A, Von Forstner C, Lützen U, Meller B, Henze E (2009) New approach for the synthesis of [18F]fluoroethyltyrosine for cancer imaging: simple, fast, and high yielding automated synthesis. Bioorg Med Chem 17:7441–7448
Hamacher K, Coenen HH (2002) Efficient routine production of the 18F-labelled amino acid O-(2-[18F]fluoroethyl)-l-tyrosine. Appl Radiat Isot 57:853–856
Vaneycken I, Blykers A, Xavier C, Caveliers V (2012) Fully automated synthesis of 18F-FET using the Synthera Platform. J Nucl Med 53(1):1476
Bourdier T, Greguric I, Roselt P, Jackson T, Faragalla J, Katsifis A (2011) Fully automated one-pot radiosynthesis of O-(2-[18F]fluoroethyl)-l-tyrosine on the TracerLab FXFN module. Nucl Med Biol 38:645–651
Hamacher K, Coenen HH, Stocklin G (1986) Efficient stereospecific synthesis of no-carrier-added 2-[18F]fluoro-2-deoxy-d-glucose using aminopolyether supported nucleophilic substitution. J Nucl Med 27:235–238
Mosdzianowski C, Lemaire C, Lauricella B, Aerts J, Morelle J-L, Gobert F (1999) Routine and multi-curie level productions of [18F]FDG using an alkaline hydrolysis on solid support. J Label Compds Radiopharm 42:515–516
Pascali C, Bogni A, Fugazza L, Cucchi C, Crispu O, Laera L, Iwata R, Maiocchi G, Crippa F, Bombardieri E (2012) Simple preparation and purification of ethanol-free solutions of 3′-deoxy-3′-[(18)F]fluorothymidine by means of disposable solid-phase extraction cartridges. Nucl Med Biol 39:540–550
Wang M, Zhang Y, Zhang Y, Yuan H (2009) Automated synthesis of hypoxia imaging agent [18F]FMISO based upon a modified Explora FDG4 module. J Radioanal Nucl Chem 280:149–155
Blom E, Koziorowski J (2014) Automated synthesis of [18F]FMISO on IBA Synthera. J Radioanal Nucl Chem 299:265–270
Wang H-E, Wu S-Y, Chang C-W, Liu R-S, Hwang L-C, Lee T-W, Chen J-C, Hwang J–J (2005) Evaluation of F-18-labeled amino acid derivatives and [18F]FDG as PET probes in a brain tumor-bearing animal model. Nucl Med Biol 32:367–375
Wang M, Yin D, Li S, Wang Y (2007) Synthesis of O-(2-[18F]fluoroethyl)-l-tyrosine and its biological evaluation in B16 melanoma-bearing mice as PET tracer for tumor imaging. Sci China Ser B-Chem 50(2):276–283
Krasikova RN, Kuznetsova OF, Fedorova OS, Maleev VI, Savel’eva TF, Belokon YN (2008) No carrier added synthesis of O-(2′-[18F]fluoroethyl)-l-tyrosine via a novel type of chiral enantiomerically pure precursor, NiII complex of a (S)-tyrosine Schiff base. Bioorg Med Chem 16:4994–5003
Krasikova RN, Orlovskaya VV, Stepanova MA, Fedorova OS (2013) The effect of reaction media and phase transfer catalyst on the fluorination yield and enantiomeric purity in asymmetric synthesis of O-(2′-[18F]fluoroethyl)-l-tyrosine. Curr Org Chem 17(19):2159–2163
Krasikova RN (2013) PET radiochemistry automation: state of the art and future trends in 18F-nucleophilic fluorination. Curr Org Chem 17(19):2097–2107
Guidance for Industry. Genotoxic and Carcinogenic Impurities in Drug Substances and Products: Recommended Approaches (2008) U.S. Department of Health and Human Services Food and Drug Administration. http://www.fda.gov/downloads/Drugs/.../Guidances/ucm079235.pdf. Accessed Dec 2008
McClatchey KD (2002) Clinical Laboratory Medicine. Lippincott Williams & Wilkins, Philadelphia, p 1693
Acknowledgments
This work was performed within the CRP program of the International Atomic Energy Agency (IAEA), contract No. 15436.
Conflict of interest
We wish to thank Scintomics GmbH, Germany, for the support of this study. The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fedorova, O., Kuznetsova, O., Stepanova, M. et al. A facile direct nucleophilic synthesis of O-(2-[18F]fluoroethyl)-l-tyrosine ([18F]FET) without HPLC purification. J Radioanal Nucl Chem 301, 505–512 (2014). https://doi.org/10.1007/s10967-014-3121-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-014-3121-2