Abstract
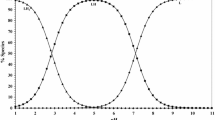
In the present study, the protonation constants of adenine (Ade), a DNA base, and of the amino acids aspartic acid, glutamic acid, asparagine, leucine, phenylalanine and tryptophan, were determined in 0.1 mol·L−1 KNO3 ionic medium and at 25.00 °C by the potentiometric method. The binary and ternary ligand complex systems that Cu(II) establishes with Ade and these amino acids were examined using the same method and in the same ionic medium; stability constants were calculated with the BEST computer software and distribution curves were drawn by means of the SPE software. The relative stability of each ternary complex was compared with that of the corresponding binary complexes in terms of their ∆log10 K values.










Similar content being viewed by others
References
Louie, A.Y., Meade, T.J.: Metal complexes as enzyme inhibitors. Chem. Rev. 99, 2711–2734 (1999)
Patel, R.N., Singh, N., Shukla, K.K., Chauhan, U.K., Chakraborty, S., Niclos-Gutierrez, J., Castineiras, A.: X-ray, spectral and biological (antimicrobial and superoxide dismutase) studies of oxalato bridged CuII–NiII and CuII–ZnII complexes with pentamethyldiethylenetriamine as capping ligand. J. Inorg. Biochem. 98, 231–237 (2004)
Fairlamb, A.H., Henderson, G.B., Cerami, A.: Trypanothione is the primary target for arsenical drugs against African tryanosomes. Proc. Natl. Acad. Sci. USA. Biochem. 86, 2607–2611 (1989)
Balcarova, Z., Kasparkova, J., Zakovska, A., Novakova, O., Sivo, M.F., Natile, G., Brabeck, V.: DNA interactions of a novel platinum drug, cis-[PtCl(NH3)2(N7-Acyclovir)]+. Mol. Pharmacol. 53, 846–855 (1998)
Lafemina, R.L., Schneider, C.L., Robbins, H.L., Callahan, P.L., Legrow, K., Roth, E., Schleif, W.A., Emini, E.A.: Requirement of active human-immunodeficiency—virus type-1 integrase enzyme for productive infection of human T-Lymhoid cells. J. Virol. 66, 7414–7419 (1992)
Moore, P.S., Jones, C.J., Mahmood, N., Evans, I.G., Goff, M., Cooper, R., Hay, A.J.: Anti-(human immunodeficiency virus) activity of polyoxotungstates and their inhibition of human–immunodeficiency-virus reverse–transcriptase. Biochem. J. 307, 129–134 (1995)
Rixe, O., Ortuzar, W., Alvarez, M., Parker, R., Reed, E., Paul, K., Fojo, T.: Oxaplatin, tetraplatin, cisplatin, and carboplatin: spectrum af activity in drug-resistant cell lines and in the cell lines of the national cancer institute’s anticancer drug screen panel. Biochem. Pharmacol. 52, 1855–1865 (1996)
Bakhtiar, R., Ochiai, E.I.: Pharmacological applications of inorganic complexes. Gen. Pharmacol. 32, 525–540 (1999)
Hammud, H.H., Nemer, G., Sawma, W., Touma, J., Barnabe, P., Bou-Mouglabey, Y., Ghannoum, A., El-Hajjar, J., Usta, J.: Copper–adenine complex, a compound, with multi-biochemical targets and potential anti-cancer effect. Chem. Biol. Interact. 173, 84–96 (2008)
Kalnayaraman, S., Krishnakumar, V., Ganesan, K.: Vibrational spectroscopic analysis of cytosine monohydrate and its copper(II) complex. Spectrochim. Acta Part A 66, 1340–1346 (2007)
Tajmir-Riahi, H.A., Langlais, M., Savoie, R.: A laser Raman spectroscopic study of the interaction of calf-thymus DNA with Cu(II) and Pb(II) ions: metal ion binding and DNA conformational changes. Nucl. Acids Res. 16, 751–762 (1988)
De la Fuente, M., Hernanz, A., Navarro, R.: IR and Raman study on the interactions of the 5′-GMP and 5′-CMP phosphate groups with Mg(II), Ca(II), Sr(II), Ba(II), Cr(III), Co(II), Cu(II), Zn(II), Cd(II), Al(III) and Ga(III). J. Biol. Inorg. Chem. 9, 973–986 (2004)
Marzilli, L.G., De Castro, B., Caradonna, J.P., Stewart, R.C., Van Vuuren, C.P.: Nucleoside complexing. A Raman and 13C NMR spectroscopic study of the binding of hard and soft metal species. J. Am. Chem. Soc. 102, 916–924 (1980)
Ghose, R.: Metal complexation with adenine and thymine. Synth. React. Inorg. Met.-Org. Chem. 22, 379–392 (1992)
Schubert, J.: Chelation in medicine. Sci. Am. 214, 40–50 (1966)
Kirschner, S., Wei, Y.K., Francis, D., Bergman, J.G.: Anticancer and potential antiviral activity of complex inorganic compounds. J. Med. Chem. 9, 369–372 (1966)
Livingstone, S.E., Nolan, J.D.: Metal chelates of biologically important compounds. I. Complexes of dl-ethionine and S-methyl-l-cysteine. Inorg. Chem. 7, 1447–1451 (1968)
Livingstone, S.E., Mihkelson, A.E.: Metal chelates of biologically important compounds. II. Nickel complexes of dialkyldithiophosphates and their adducts with nitrogen heterocycles. Inorg. Chem. 9, 2545–2551 (1970)
Rosenberg, B., Vancamp, L., Trosko, J.E., Mansour, V.H.: Platinum compounds: a new class of potent antitumor agents. Nature 222, 385–386 (1969)
Helene, C., Maurizot, J.C.: Interactions of oligopeptides with nucleic acids. CRC Crit. Rev. Biochem. 10, 213–258 (1981)
Bere, A., Helene, C.: Formation of ternary complexes involving zinc or copper ions, polynucleotides, and polypeptides containing glutamic acid and tyrosine residues. Biopolymers 18, 2659–2672 (1979)
Masoud, M.S., Heiba, A.M., Ashmawy, F.M.: Synthesis and characterization of barbituric and thiobarbituric acid complexes. Trans. Met. Chem. 8, 124–126 (1983)
Masoud, M.S., El-Merghany, A., Abd El-Kaway, M.Y.: Synthesis and physico-chemical properties of biologically active purine complexes. Syn. React. Inorg. Met. Org. Nano Met. Chem. 39, 537–553 (2009)
Zaworotko, M.T., Hammud, H.H., Mc-Manus, G.J., Ghannoum, A.M., Kabbani, A., Masoud, M.: Synthesis and characterization of some transition metal complexes with mixed adenine and acetylacetonate ligands: crystal structures of solvated complex {[Cu(acac)2(adenine)]·EtOH}and{[Cu(acac)2(adenine)]·DMF·H2O}. J. Chem. Crystallogr. 39, 853–863 (2009)
Masoud, M., Awad, M., Shaker, M., El-Tahawy, M.M.T.: The role of structural chemistry in the inhibitive performance of some aminopyrimidines on the corrosion of steel. Corros. Sci. 52, 2387–2396 (2010)
Williams, D.R.: The Metals of Life. Von Nostrand Reinhold, London (1971)
Sigel, H.: Coordination Chemistry, vol. 20. Pergamon Press, Oxford (1980)
Gran, G.: Determination of equivalent point in potentiometric titrations. Acta Chem. Scand. 4, 559–577 (1950)
Serjant, E.P.: Potentiometry and Potentiometric Titrations. Wiley, New York (1984)
Gran, G.: Gran plots. Analyst 7, 661–671 (1952)
Rossotti, H.: Chemical Applications of Potentiometry. Van Nostrand, London (1968)
Schwarzanbach, G., Flaschka, H.: Complexometric Titrations. Methuen, New York (1969)
Perin, D.D., Amerago, W.L.F.: Purification of Laboratory Chemicals, 1st edn, p. 148. Pergamon Press, Oxford (1966)
Türkel, N.: Stability of metal chelates of some hydroxamic acid ligands. J. Chem. Eng. Data 56, 2337–2342 (2011)
Chalmers, R.A.: Chemistry of Complex Equilibria. Von Nostrand Reinhold Company, London (1970)
Martell, A.E., Motekaitis, R.J.: Determination and Use of Stability Constants. VCH Publishers, New York (1989)
Zimmer, S., Biltonen, R.: The thermodynamics of proton dissociation of adenine. J. Solution Chem. 1, 291–298 (1972)
Demir, H.D., Pekin, M., Cücü, A.K., Dölen, E., Aboul-Enein, H.Y.: Potentiometric studies of mixed complexes of cobalt(II) and copper(II) with l-asparagine and adenine. Toxicol. Environ. Chem. 71, 357–367 (1999)
de Miranda, J.L., Felcman, J.: Study on guanidino–scarboxylate interactions in copper(II) ternary complexes of guanidino acetic acid with glutamic and aspartic acids. Polyhedron 22, 225–233 (2003)
Sajadi, S.A.A.: Metal ion binding properties of l-glutamic acid and l-aspartic acid, a comparative investigation. Nat. Sci. 2, 85–90 (2010)
Sovago, I., Kiss, T., Gergely, A.: Critical survey of stability constants of complexes of aliphatic amino acids. Pure Appl. Chem. 65, 1029–1080 (1993)
Anderson, K.P., Greenhalgh, W.O., Izatt, R.M.: Formation constants and enthalpy and entropy values for the association of H+ and Cu2+ with glycinate and phenylalanate ions in aqueous solution at, 10, 25, and 40°. Inorg. Chem. 5, 2106–2109 (1966)
Sajadi, S.A.: Complex binding behaviour of l-tryptophan and related amino acids, a comperative investigation. Am. J. Chem. 1, 60–64 (2011)
Ammar, R.A., Al-Mutiri, E., Abdalla, M.A.: Equilibrium study of the mixed complexes of copper(II) with adenine and amino acids in aqueous solution. J. Solution Chem. 39, 727–737 (2010)
Harkins, T.R., Freiser, H.: Adenine–metal complexes. J. Am. Chem. Soc. 80, 1132–1135 (1958)
Hamada, Y.Z., Burkey, T., Waddell, E., Aitha, M., Phambu, N.: Reactions of Zn+2, Cd+2 and Hg+2 with free adenine. J. Appl. Solution Chem. Model. 2, 77–84 (2013)
Aliyu, H.N., Náaliya, J.: Potentiometric studies on essential metal(II) amino acid complexes. Int. Res. J. Pharm. Pharmacol. 2, 076–080 (2012)
Lahsasni, S.A., Ammar, R.A., Amin, M.F., Shoukry, E.M.: Mixed ligand complex formation of Cu(II) with 1,2-diphenylethylenediamine as primary ligand and amino acids as secondary ligands. Int. J. Electrochem. Sci. 7, 7699–7711 (2012)
Yamauchi, O., Odani, A.: Structure–stability relationship in ternary copper(II) complexes involving aromatic amines and tyrosine or related amino acids. Intramolecular aromatic ring stacking and its regulation through tyrosine phosphorylation. J. Am. Chem. Soc. 107, 5938–5945 (1985)
Hallman, P.S., Perin, D.D., Watt, A.E.: The computed distribution of copper(II) and zinc(II) ions among seventeen amino acids present in human blood plasma. Biochem. J. 121, 549–555 (1971)
Malini-Balaksrishnan, R., Sheller, K.H., Haering, U.K., Tribolet, R., Sigel, H.: Ternary complexes in solution. 45. Intramolecular aromatic ring stacking interactions in dependence on the ligand structure, geometry of the coordination sphere of the metal ion, and solvent composition. Inorg. Chem. 24, 2067–2076 (1985)
Sigel, H.: Metal Ions in Biological Systems: Mixed Ligand Complexes, vol. 2. Marcel Decker Inc., New York (1973)
De Witt, R., Watters, J.I.: Spectrophotometric investigation of a mixed complex of copper(II) ion with oxalate ion and ethylenediamine. J. Am. Chem. Soc. 76, 3810 (1954)
Khalil, M.M., Radalla, A.E., Qasem, F., Khaled, R.: Equilibrium studies of ternary systems containing some selected transition metal ions, triazoles and aromatic carboxylic acids. Korean J. Chem. Eng. 31, 109–119 (2014)
Acknowledgments
We acknowledge The Scientific and Technical Research Council of Turkey (TUBITAK) for financial support to 113Z/600 project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Türkel, N. Equilibrium Study of the Mixed Complexes of Copper(II) with Adenine and Amino Acids in Aqueous Solution. J Solution Chem 44, 1267–1280 (2015). https://doi.org/10.1007/s10953-015-0344-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-015-0344-y