Abstract
The aim of this work is to study the preparation and characterization of a new nanocomposite which consists of chemically-modified multiwall carbon nanotubes covered by randomly-deposited nanoparticles of hematite. The morphology, structural and physical properties of the investigated nanomaterial were determined by means of transmission electron microscopy, X-ray diffraction and vibrating sample magnetometry at ambient conditions. The presence of residual catalyst nanospheres inside multiwall carbon nanotubes was confirmed by transmission electron microscopy. The signal coming from this contamination was under the detection limit of X-ray diffractometer, therefore it was not registered.
Similar content being viewed by others
1 Introduction
In 1991, Iijima managed to prepare the first multiwall carbon nanotubes (MWCNTs) via arc discharge process [1]. Since then, carbon nanotubes (CNTs) became the source of advanced studies in the field of physics, chemistry and material sciences. At the same time, CNTs started to play a significant role in numerous applications in many fields including: medicine [2], high-performance adsorbents [3], sector of energy storage and conversion [4, 5], etc.
There are four commonly-used CNTs fabrication techniques: arc discharge [1], laser ablation [6], chemical vapor deposition (CVD) [7] and vapor–liquid–solid (VLS) method [8]. However, the two latter techniques seem to have certain advantages over the other. For instance, they are not high temperature processes. The growth and dimensions of CNTs (via CVD or VLS) can be easily-controlled by fitting reaction parameters such as: temperature, source of carbon, or catalyst concentration. However, CNTs growth complications arise when using metallic catalysts such as iron (Fe), cobalt (Co) or nickel (Ni) because they form metal carbides together with carbon precursors which become the seeds for carbon nanotubes growth. Therefore, they often remain built into the tubes and become an integral part of the nanomaterial.
So far, it has been reported that the purification of contaminated CNTs could be achieved via the functionalization of carbon nanotubes [9]. Through this procedure, it is possible to cover the CNTs surface by various chemical groups such as: -OH, -COOH, COONH4, etc. Furthermore, it has been proven that the traces of catalyst are gradually removed from the nanotubes at the same time [10].
Another material which is interesting in the case of this work is hematite (α-Fe2O3). This oxide belongs to the group of low-cost and environmentally-friendly transition metal oxides. Moreover, α-Fe2O3 is the most thermodynamically stable iron oxide and it is extensively used in the production of pigments, jewelry, catalysts, sensors, hard and soft magnets, devices for energy storage and conversion, etc. [11].
The purpose of this work is to synthesize and characterize a new nanocomposite which consists of chemically-modified multiwall carbon nanotubes (MWCNTs) covered by randomly-deposited nanoparticles of hematite (α-Fe2O3). Such nanomaterial promises to combine the features of both MWCNTs and α-Fe2O3 making it an useful material in many fields for future applications.
2 Experimental Details
The raw multiwall carbon nanotubes (MWCNTs; 93 % of purity; CNT CO., Ltd. from South Korea) were manufactured via CVD process with iron (Fe) as the catalyst. Such MWCNTs were chemically-modified by heating at 120 °C in concentrated nitric acid (68 % HNO3) for 50 h under a reflux. Then, they were filtered and washed with distilled water until the pH value of the filtrate reached 6. Thereby, the MWCNTs were oxidized and formed carboxylic groups attached to the ends and the walls of CNTs (MWCNTs-COOH) [12]. Such functionalized CNTs were treated with a 25 % aqueous solution of ammonia and then rinsed with distilled water during filtration to obtain the ammonium salt (MWCNTs-COONH4). After that, 1 g of MWCNTs-COONH4 was dispersed in 500 ml of distilled water and cooled to 4 °C. In two other beakers 0.215 g of iron dichloride tetrahydrate (FeCl2⋅ 4H2O; 98 % of purity; Sigma-Aldrich) and 0.588 g of iron trichloride hexahydrate (FeCl3⋅ 6H2O; 97 % of purity; Sigma-Aldrich) were dissolved in distilled water and added by droplets to the previously prepared carbon nanotube dispersion mixed with a magnetic stirrer. After 30 min, the pH of dispersion was brought up to 10 with 1 M NaOH. The obtained product was heated up to 100 °C on a hot plate with magnetic stirring for 30 min. Finally, the prepared nanocomposite was centrifuged and washed with distilled water until the value of pH reached 7.
The obtained nanomaterial was studied using a Phillips X’Pert diffractometer (XRD) equipped with a Cu X-ray source and a parallel beam Bragg reflection mirror, a JEOL JEM 3010 transmission electron microscope (TEM) and an Oxford Instruments Ltd. vibrating sample magnetometer (VSM) in the range of magnetic field between −0.6 T and 0.6 T. The XRD and VSM measurements were performed under ambient conditions.
Additionally, the results for commercially-available reference samples of α-Fe2O3 powder (99.98 % of purity; Carl Roth GmbH) and the chemically-modified multiwall carbon nanotubes (MWCNTs-COONH4) are presented in this work.
3 Results and Discussion
TEM images of chemically-modified MWCNTs and α-Fe2O3/MWCNTs nanocomposite are presented in Fig. 1a and 1c. A residual amount of catalyst coming from the preparation process can also be seen built into the nanotube (Fig. 1b). TEM studies show that MWCNTs are very disordered, braided, and are successfully coated by the randomly-dispersed hematite nanoparticles. The diameter of CNTs varies from 10 to 40 nm. The average diameters of α-Fe2O3 and traces of catalyst are approximately 50 nm and 12 nm, respectively.
XRD has great advantages in comparison with the other experimental techniques because it is simple in terms of implementation and it also has the ability of measuring for a long period of time without any negative effects on the sample. Therefore, the XRD technique is a good tool to study the structure of α-Fe2O3/MWCNTs nanocomposite and reference samples. These results are presented in Fig. 2. Recorded XRD patterns confirm that MWCNTs are successfully coated by hematite. The positions of peaks originating from α-Fe2O3 and the chemically-modified MWCNTs lie at the same angle positions for all structures. Moreover, no significant changes in the functionalized MWCNTs structure after deposition of hematite are observed.
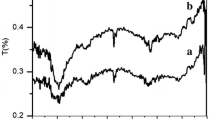
Figure 3 presents the magnetization hysteresis loops of MWCNTs-COONH4 and α-Fe2O3/MWCNTs. The differences in curve shapes indicate different magnetic behaviors. The functionalized MWCNTs contain the encapsulated traces of catalyst coming from the CVD process. The previous studies performed on this nanomaterial have shown that the residual amount of catalyst is associated with the presence of non-stoichiometric iron carbide (Fe x C), α-Fe and ferrihydrite [10]. Most of these iron compounds are ferromagnetic, therefore magnetization hysteresis of MWCNTs-COONH4 reveals the ferromagnetic behavior. On the other hand, the shape of the magnetization curve for the studied nanocomposite exhibits more complex behavior with a coercivity and large high field susceptibility. It is related to the strong influence of α-Fe2O3 deposited on the surface of carbon nanotubes being superimposed on the ferromagnetic behavior of the catalyst residue, of which the content in the investigated material is much lower than hematite. The recorded values of saturated magnetization (M S), remnant magnetization (M R) and coercivity (H C) for MWCNTs-COONH4 are equal 0.26 emu/g, 0.10 emu/g and 0.04 T (400 Oe), respectively. For α-Fe2O3/MWCNTs, the remnant magnetization equals 0.11 emu/g and the coercivity equals 0.03 T (300 Oe). Taking into account that all magnetic moment values are given in this work per unit of total mass (emu/g), considering all compounds inside and outside of CNTs and also the nanotubes mass, it is very difficult to perform further analysis of the obtained results. Nevertheless, the presence of encapsulated catalyst traces in CNTs and diamagnetic contribution of the graphitic nanotubes play important roles for the magnetic properties of both MWCNTs-COONH4 and α-Fe2O3/MWCNTs.
4 Conclusion
This work confirms that α-Fe2O3/MWCNTs nanocomposite could be simply prepared via the proposed chemical method. Morphology and structural and magnetic features of this material were determined. Moreover, the investigated nanomaterial is interesting due to the variety of possible applications; for example in the lithium-ion batteries [4, 13] and/or the supercapacitors [5, 14]. Therefore, it is supposed to be a very perspective material with regards to future research.
References
Iijima, S.: Nature 354, 56–58 (1991)
Liu, Z., Chen, K., Davis, C., Sherlock, S., Cao, Q., Chen, X., Dai, H.: Cancer Res. 68, 6652–6660 (2008)
Li, Y.H., Wang, S.G., Zhang, X.F., Wei, J.Q., Xu, C., Luan, Z.K., Wu, D.H.: Mater. Res. Bull. 38, 469–476 (2003)
de las Casas, C., Li, W.Z.: J. Power Sources 208, 74–85 (2012)
Pan, H., Li, J., Feng, Y.: Nanoscale Res. Lett. 5, 654–668 (2010)
Zhang, Y., Gu, H., Iijima, S.: Appl. Phys. Lett. 73, 3827–3829 (1998)
Kong, J., Soh, H.T., Cassell, A.M.: Nature 395, 878–881 (1998)
Kukovitsky, E.F., L’vov, S.G., Sainov, N.A.: Chem. Phys. Lett. 317, 65–70 (2000)
Jamrozik, A., Mazurkiewicz, M., Malolepszy, A., Stobinski, L., Matlak, K., Korecki, J., Kurzydłowski, K.J., Burda, K.: Phys. Status Solidi A-Appl. Mat. 208, 1783–1786 (2011)
Kuryliszyn-Kudelska, I., Małolepszy, A., Mazurkiewicz, M., Stobinski, L., Kurzydłowski, K.J., Dobrowolski, W.: Phys. Status Solidi A-Appl. Mat. 208, 1787–1790 (2011)
Cornell, R.M., Schwertmann, U.: The iron oxides. structure, properties, reactions, occurrences and uses: structure, properties, reactions, occurrences and uses. Wiley-VCH, Weinheim (2003)
Stobinski, L., Lesiak, B., Kover, L., Toth, J., Biniak, S., Trykowski, G., Judek, J.: J. Alloys Compd. 501, 77–84 (2010)
Hanga, B.T., Watanabe, I., Doi, T., Okada, S., Yamaki, J.I.: J. Power Sources 161, 1281–1287 (2006)
Xie, K.Y., Li, J., Lai, Y.Q., Lu, W., Zhang, Z., Liu, Y.X., Zhou, L.M., Huang, H.T.: Electrochem. Commun. 13, 657–660 (2011)
Acknowledgments
This work was supported by the Foundation for Polish Science International PhD Projects Programme co-financed by the EU European Regional Development Fund, A.M. thanked the support from the European Union funds by the European Social Fund and L.S. thanked the National Center for Research and Development for support by the project no. PBS1/A5/15/2012. Sincere thanks towards Dr. J. Syzdek for the research consultations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Krajewski, M., Malolepszy, A., Stobinski, L. et al. Preparation and Characterization of Hematite-Multiwall Carbon Nanotubes Nanocomposite. J Supercond Nov Magn 28, 901–904 (2015). https://doi.org/10.1007/s10948-014-2794-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-014-2794-7