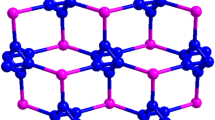
Abstract
The reaction of N,N,N′,N′-tetramethylethylenediamine (tmeda) and NiCl2 with the soft, Lewis acidic Hg(CN)2 and HgCl2 in ethanol formed the 2-D layer structure {(tmeda)Ni[Hg(CN)2]2}[HgCl4] (1), isostructural to the Cu(II) analogue. Complex 1 crystallizes in the tetragonal, non-centric \(P \overline{4} {\text{ }}2_{1}\) m space group and contains a 2-D cationic layer of {(tmeda)Ni[Hg(CN)2]2}2+ units in which the six-coordinate Ni(II) centres are bridged by four Hg(CN)2 groups and capped by a tmeda ligand. This array is interspersed with a layer of [HgCl4]2− anions, which form bridging Hg–Cl bonds with the Hg(CN)2 units. The formation of 1 is very sensitive to reaction conditions; the addition of water to the mixture yields the related “structural pitstop” 2-D array {(tmeda)Ni(H2O)[Hg(CN)2]}{[Hg(CN)Cl]2Cl2}·H2O (2), in which the halide migration among Hg(II) centres is incomplete. The larger zero-field splitting D-values of 6.91(1) cm−1 for 1 vs. 2.85(4) cm−1 for 2 indicate that some weak antiferromagnetic interactions are likely present in 1. The reaction of tmeda/Cu(ClO4)2·6H2O with Hg(CN)2 yields [Cu(tmeda)(μ-OH)(ClO4)]2[Hg(CN)2(H2O)2][Hg(CN)2] (3) which is composed of [Cu(tmeda)(μ-OH)(ClO4)]2 dimers in which the \(\hbox{ClO}_4^{-}\) anions cis-bridge the copper(II) centres in the axial positions as well as bind to two adjacent Hg(CN)2 moieties; the perchlorate anion is acting as a rare η4–μ4–ClO4 ligand. N-cyano interactions also exist between the Hg(II) centres; overall, a 2-D corrugated sheet structure which stacks via Cl–O–Hg bridges to yield a 3-D array is formed. The χM T value for 3 decreases with decreasing temperature; a maximum in χM vs. T at 20 K is also observed. This is consistent with antiferromagnetic interactions within the copper(II) dimer, which were fit with the Bleaney-Bowers model to yield J=−23.1(1) cm−1, g=2.113(5) and a paramagnetic impurity P=0.017(1).




Similar content being viewed by others
References
K. R. Dunbar and R. A. Heintz, Prog. Inorg. Chem. 45, 283 (1997) and references therein; J. Lefebvre and D. B. Leznoff, in Macromolecules Containing Metal and Metal-like Elements, A. Abd-El-Aziz, C. E. Carraher, C. U. Pittman, J. E. Sheats, and M. Zeldin, eds. (John Wiley and Sons, New York, 2005) Vol. 5
M. Verdaguer, A. Bleuzen, V. Marvaud, J. Vaissermann, M. Seuleiman, C. Desplanches, A. Scuiller, C. Train, R. Garde, G. Gelly, C. Lomenech, I. Rosenman, P. Veillet, C. Cartier and F. Villain, Coord. Chem. Rev. 190–192, 1023 (1999)
M. Ohba and H. Ôkawa, Coord. Chem. Rev. 198, 313 (2000)
J. S. Miller Inorg. Chem. 39, 4392 (2000)
T. Iwamoto, in Comprehensive Supramolecular Chemistry, J. M. Lehn, J. L. Atwood, J. E. D. Davies, D. D. MacNicol, F. Vögtle, G. Alberti, and T. Bein, eds. (Pergamon Press, Oxford, 1996)
D. B. Leznoff, B.-Y. Xue, B. O. Patrick, V. Sanchez, and R. C. Thompson, Chem. Commun. 259 (2001)
D. B. Leznoff, B.-Y. Xue, C. L. Stevens, A. Storr, R. C. Thompson, and B. O. Patrick, Polyhedron 20, 1247 (2001)
D. B. Leznoff, B.-Y. Xue, R. J. Batchelor, F. W. B. Einstein, and B. O. Patrick, Inorg. Chem. 40, 6026 (2001)
J. Lefebvre, R. J. Batchelor, and D. B. Leznoff, J. Am. Chem. Soc. 126, 16117 (2004)
J. Lefebvre and D. B. Leznoff, Gold Bull. 38, 47 (2005)
C. J. Shorrock, B.-Y. Xue, P. B. Kim, R. J. Batchelor, B. O. Patrick, and D. B. Leznoff, Inorg. Chem. 41, 6743 (2002)
N. D. Draper, R. J. Batchelor, B. C. Sih, Z.-G. Ye, and D. B. Leznoff, Chem. Mater. 15, 1612 (2003)
N. D. Draper, R. J. Batchelor, and L. B. Leznoff, Cryst. Growth Des. 4, 621 (2004)
N. D. Draper, R. J. Batchelor, P. M. Aguiar, S. Kroeker, and D. B. Leznoff, Inorg. Chem. 43, 6557 (2004)
C. Janiak, Dalton Trans. 2781 (2003)
D. J. Chesnut, D. Hagrman, P. J. Zapf, R. P. Hammond, R. LaDuca, R. C. Haushalter, and J. Zubieta, Coord. Chem. Rev. 192, 737 (1999)
I. J. Hodgkinson and Q. H. Wu, Birefringent Thin Films and Polarizing Elements, World Scientific Publ., River Edge (1997); J. P. Lesso, A. J. Duncan, W. Sibbett, and M. J. Padgett, Appl. Opt. 39, 592 (2000)
V. H. Crawford, H. W. Richardson, J. R. Wasson, D. J. Hodgson, and W. E. Hatfield, Inorg. Chem. 15, 2107 (1976)
O. Kahn, Molecular Magnetism (VCH, Weinheim, 1993)
E. J. Gabe, P. S. White, and G. D. Enright DIFRAC: A Fortran 77 Control Routine for 4-Circle Diffractometers (N. R. C., Ottawa, 1995)
E. J. Gabe, Y. LePage, J. -P. Charland, F. L. Lee, and P. S. White, J. Appl. Crystallogr. 22, 384 (1989)
P. W. Betteridge, J. R. Carruthers, R. I. Cooper, C. K. Prout, and D. J. Watkins, J. Appl. Cryst. 36, 487 (2003)
(a) International Tables for X-ray Crystallography, Vol. IV, Kynoch Press, Birmingham (present distributor Kluwer Academic Publishers: Boston, MA) (1974) p. 99
R. A. Penneman and L. H. Jones J. Inorg. Nucl. Chem. 20, 19 (1961)
D. Grdenic, Quart. Rev. 19, 303 (1965)
W. E. Hatfield, in Magneto-Structural Correlations in Exchange Coupled Systems W.R. Dillett, D. Gatteschi, and O. Kahn, eds. (Reidel, Dordrecht, 1984), pp. 555
F. Haftbaradaran, D. B. Leznoff, and V. E. Williams, Dalton Trans. 2105 (2003)
H. Oshio, T. Watanabe, A. Ohto, T. Ito, and U. Nagashima, Angew. Chem., Int. Ed. Engl. 33, 670 (1994)
H. Oshio, T. Watanabe, A. Ohto, T. Ito, T. Ikoma, and S. Tero-Kubota, Inorg. Chem. 36, 3014 (1997)
B. Bleaney and K. D. Bowers, Proc. R. Soc. London A 214, 451 (1952)
E. Ruiz, P. Alemany, S. Alvarez, and J. Cano, J. Am. Chem. Soc. 119, 1297 (1997)
E. Ruiz, P. Alemany, S. Alvarez, and J. Cano, Inorg. Chem. 36, 3683 (1997)
H. Hu, Y. Liu, D. Zhang, and C. Liu, J. Mol. Struct. 546, 73 (2001)
K. T. McGregor, N. T. Watkins, D. L. Lewis, R. F. Drake, D. J. Hodgson, and W. E. Hatfield, Inorg. Nucl. Chem. Lett. 9, 423 (1973)
G. A. van Albada, I. Mutikainen, U. Turpeinen, and J. Reedijk, Inorg. Chim. Acta 324, 273 (2001) and references therein
I. Castro, J. Faus, M. Julve, C. Bois, J. A. Real, and F. Lloret, J. Chem. Soc., Dalton Trans. 47 (1992)
Acknowledgments
Financial support from NSERC (D.B.L.) and Natural Resources Canada (N.D.D., M.J.K.) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Special Issue to honour Professor Richard J. Puddephatt
Rights and permissions
About this article
Cite this article
Draper, N., Katz, M., Batchelor, R. et al. Structural Pitstops and Turnoffs on the Way to the Birefringent 2-D Layer Structure \(\{\hbox{(tmeda)M[Hg(CN)}_{2}]_{2}\}[\hbox{HgCl}_{4}]\) (M=Cu, Ni). J Inorg Organomet Polym 15, 447–458 (2005). https://doi.org/10.1007/s10904-006-9017-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-006-9017-z