Abstract
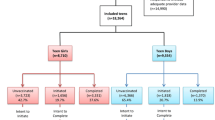
The objective of this study was to examine correlates of human papillomavirus (HPV) vaccine use according to Advisory Committee on Immunization Practices (ACIP)’s recommendations among US adolescent girls. We used National Immunization Survey of Teens 2013 data. Based on provider-verified (n = 9403) information, 57.3, 39.1 and 19.0 % of adolescent girls, initiated, completed and completed the HPV vaccine according to ACIP’s recommendation (by age 12), respectively. Hispanic race/ethnicity, a physician recommendation for HPV vaccine and ≥1 influenza vaccine in the past 3 years were all associated with a higher likelihood of compliance with ACIP’s recommendation. Girls from a larger family and those whose immunization provider was a STD/school/teen clinic were less likely to receive the vaccine at the recommended age compared to a girl raised in a smaller sized family and received immunization from a hospital facility, respectively. Only one-fifth of 13–17 yo girls receive the HPV vaccine by age 12 as recommended by ACIP. Physician visits and influenza vaccination settings are opportunities to improve vaccine series completion at the recommended age.

Similar content being viewed by others
References
Garland, S. M., Steben, M., Sings, H. L., et al. (2009). Natural history of genital warts: analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. Journal of Infectious Diseases, 199(6), 805–814.
Bosch, F. X., Burchell, A. N., Schiffman, M., et al. (2008). Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine, 26(Suppl 10), K1–K16.
Joura, E. A., Giuliano, A. R., Iversen, O. E., et al. (2015). A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. New England Journal of Medicine, 372(8), 711–723.
Reagan-Steiner, S., Yankey, D., Jeyarajah, J., et al. (2015). National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 Years: United States, 2014. MMWR Morbidity Mortality Weekly Report, 64(29), 784–792.
Hopkins, T. G., & Wood, N. (2013). Female human papillomavirus (HPV) vaccination: Global uptake and the impact of attitudes. Vaccine, 31(13), 1673–1679.
Markowitz, L. E., Dunne, E. F., Saraiya, M., Centers for Disease Control and Prevention (CDC). et al. (2014). Human papillomavirus vaccination: Recommendations of the advisory committee on immunization practices (ACIP). MMWR Recommendation Report, 63(RR-05), 1–30.
Lehtinen, M., Paavonen, J., Wheeler, C. M., et al. (2012). Overall efficacy of HPV-16/18 AS04 adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncology, 13(1), 89–99.
Block, S. L., Nolan, T., Sattler, C., et al. (2006). Comparison of the immunogenicity and reactogenicity of a prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in male and female adolescents and young adult women. Pediatrics, 118(5), 2135–2145.
Pedersen, C., Petaja, T., Strauss, G., et al. (2007). Immunization of early adolescent females with human papillomavirus type 16 and 18 L1 virus-like particle vaccine containing AS04 adjuvant. Journal of Adolescent Health, 40(6), 564–571.
Dunne, E. F., Unger, E. R., Sternberg, M., et al. (2007). Prevalence of HPV infection among females in the United States. Journal of American Medical Association, 297(8), 813–819.
Rahman, M., McGrath, C. J., Hirth, J. M., & Berenson, A. B. (2015). Age at HPV vaccine initiation and completion among US adolescent girls: Trend from 2008 to 2012. Vaccine, 33(5), 585–587.
Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases, National Center for Health Statistics. (2013). National immunization survey-teen. A user’s guide for the 2011 public use data file. Retrieved from http://ftp.cdc.gov/pub/Health_Statistics/NCHS/Dataset_Documentation/NIS/NISPUF13_DU.pdf.
Jain, N., Singleton, J. A., Montgomery, M., & Skalland, B. (2009). Determining accurate vaccination coverage rates for adolescents: The National Immunization Survey-Teen 2006. Public Health Reports, 124(5), 642–651.
Kahn, J. A., Ding, L., Huang, B., Zimet, G. D., Rosenthal, S. L., & Frazier, A. L. (2009). Mothers’ intention for their daughters and themselves to receive the human papillomavirus vaccine: a national study of nurses. Pediatrics, 123(6), 1439–1445.
Daley, M. F., Crane, L. A., Markowitz, L. E., et al. (2010). Human papillomavirus vaccination practices: A survey of US physicians 18 months after licensure. Pediatrics, 126(3), 425–433.
Vadaparampil, S. T., Kahn, J. A., Salmon, D., et al. (2010). Missed clinical opportunities: Provider recommendations for HPV vaccination for 11–12 year old girls are limited. Vaccine, 29(47), 8634–8641.
Dempsey, A., Cohn, L., Dalton, V., & Ruffin, M. (2010). Patient and clinic factors associated with adolescent human papillomavirus vaccine utilization within a university-based health system. Vaccine, 28(4), 989–995.
Funding
Dr. Hirth is supported by a research career development award (K12HD052023: Building Interdisciplinary Research Careers in Women’s Health Program—BIRCWH, PI: Berenson) from the Office of Research on Women’s Health (ORWH), the Office of the Director (OD), the National Institute of Allergy and Infectious Diseases (NIAID), and NICHD at the National Institutes of Health.
Author Contributions
MR contributed toward the conception and design of the study, conducted data analysis, drafted and revised the manuscript and approved the final version. JMH and ABB contributed toward introduction and discussion, revised the manuscript and approved the final version.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
None reported.
Rights and permissions
About this article
Cite this article
Rahman, M., Hirth, J.M. & Berenson, A.B. Adherence to ACIP Recommendation for Human Papillomavirus Vaccine Among US Adolescent Girls. J Community Health 42, 385–389 (2017). https://doi.org/10.1007/s10900-016-0267-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10900-016-0267-6