Abstract
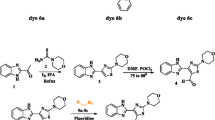
The synthesis and solvatochromic behavior of four novel carbazole based fluorescent styryl dyes were explained. In chlorinated solvents such as DCM and chloroform, these dyes show bathochromic shift in their absorption as well as emission. The styryl dyes 6b and 6c show solid state yellow fluorescence. DFT and TD-DFT computations were performed to study structural, molecular, electronic and photophysical properties of these dyes. The computed absorption and emission wavelength values are found to be in good agreement with the experimental results. The photophysical properties of these 1-styryl carbazole dyes are also compared with the recently reported 3-styrl carbazole dyes. The unique behavior of dye 6d is well explained by its optimized geometry found in the excited state. Ratio of ground to excited state dipole moment of the synthesized novel styryl compounds were calculated by Bakhshiev and Bilot-Kawski correlations.














Similar content being viewed by others
References
Chilton JA, Goosey MT (1995) Special Polymers for Electronics and Optoelectronics. Champman and Hall, London
Law KY (1993) Chem Rev 93:449–486
Krotkus S, Kazlauskas K, Miasojedovas A, Gruodis A, Tomkeviciene A, Grazulevicius JV, Jursenas SJ (2012) Phys Chem C 116:7561–7572
Jiang W, Duan L, Qiao J, Dong G, Zhang D, Wang L, Qiu YJ (2011) Mater Chem 21:4918–4926
Hsieh BR, Litt MR (1985) Macromolecules 18:1388–1394
Park JH, Koh T, Do Y, Lee MH, Yoo SJ (2012) Poly Sci Part A 50:2356–2365
Oshima R, Uryu T, Seno M (1985) Macromolecules 18:1043–1045
Uryu T, Ohkawa H, Oshima R (1987) Macromolecules 20:712–716
Shattuck MD (1969) Vahtra. U US Patent 3:484,327
Hu CJ, Oshima R, Sato S, Seno MJ (1988) Polym Sci C: Polym Lett 26:441–450
Ho MS, Barrett C, Paterson J, Esteghamatian M, Natansohn A, Rochon P (1996) Macromolecules 29:4613–4618
V. D. Gupta: A. B. Tathe; V. S. Padalkar; V. S. Patil; K. R. Phatangare; P. G. Umape; N. Sekar Journal of fluorescence 2013, pp 1-18.
Shen J, Yang X, Huang T, Lin JT, Ke T, Chen W (2007) C.; Ming-Chang P. Yeh. Adv Funct Mater 17:983–995
Li, L.; Yuan, N.; Wang, P.; Wu, Y.; Song, Y.; Chen, Z.; He, C. J. Phys. Org. Chem. 2012, 2937-2944.
Yang Z, Chi Z, Xu B, Li H, Zhang X, Li X, Liu S, Zhang Y, Xu JJ (2010) Mater Chem 20:7352–7359
Leclerc N, Michaud A, Sirois K, Morin JF, Leclerc M (2006) Adv Funct Mater 16:1694–1704
Blouin N, Michaud A, Leclerc M (2007) Adv Mater 19:2295–2300
Blouin N, Michaud A, Gendron D, Wakim S, Blair E, Neagu-Plesu R, Belletête M, Durocher G, Tao Y, Leclerc MJ (2008) Am Chem Soc 130:732–742
Zou Y, Gendron D, Badrou-Aïch R, Najari A, Tao Y, Leclerc M (2009) Macromolecules 42:2891–2894
Park SH, Roy A, Beaupré S, Cho S, Coates N, Moon JS, Moses D, Leclerc M, Lee K, Heeger AJ (2009) Nat Photonics 3:297–303
Lai H, Hong J, Liu P, Yuan C (2012) Li. Y.; Fang, Q. RSC Advances 2:2427–2432
Wakim S, Beaupré S, Blouin N, Aich B, Rodman S, Gaudiana R, Tao Y, Leclerc MJ (2009) Mater Chem 19:5351–5358
Chang CC, Kuo IC, Lin JJ, Lu YC, Chen CT, Back HT, Lou PJ, Chang TC (2004) Chem Biodivers 1:1377–1384
Fei X, Gu Y, Li C, Yang XJ (2012) Fluoresc 22:807–814
Lu M, Zhu Y, Ma K, Cao L, Wang K (2012) Spectrochim Acta A Mol Biomol Spectrosc 95:128–134
Zhang Q, Gao Y, Zhang S, Wu J, Zhou H, Yang J (2012) Taob.; Tia, Y. Dalton Trans 41:7067–7072
Eum SJ, Kwon HJ, Kim SM, Yoon SS (2011) WO 2001/105700 A1
Ramkumar S, Manoharan S, Anandan S (2012) Dyes Pigm 94:503–511
Li L, Wu Y, Zhou Q, Chunying He CJ (2012) Phys Org Chem 25:362–372
Yang Z, Zhao N, Sun Y, Miao F, Liu Y, Liu X, Zhang Y, Ai W, Song S, Shen X, Yu X, Sun J, Wong W (2012) Chem Commun 48:3442–3444
Gupta VD, Padalkar VS, Phatangare KR, Patil VS, Umape PG, Sekar N (2011) Dyes Pigm 88:378–384
Gupta VD, Tathe AB, Padalkar VS, Patil VS, Phatangare KR, Umape PG, Ramasami P.Sekar NJ (2012) Fluoresc 22:807–814
Vidya S, Ravikumar C, Hubert JI, Kumaradhas P, Devipriyac B, Raju K. Vibrational spectra and structural studies of nonlinear optical crystal ammonium D, L-tartrate: a density functional theoretical Approach. J Raman Spectrosc 2011; 42:676e84.
Treutler O, Ahlrichs R. Efficient molecular numerical integration schemes. J Chem Phys 1995; 102:346e54.
Hehre WJ, Radom L (1986) Schleyer PvR, Pople J. Ab initio molecular orbital theory. Wiley, New York
Bauernschmitt R, Ahlrichs R. Treatment of electronic excitations within the adiabatic approximation of time dependent density functional theory. ChemPhys Lett 1996; 256:454e64
Furche F, Rappaport D (2005) Density functional theory for excited states: equilibrium structure and electronic spectra. In: Olivucci M (ed) Compu-tational Photochemistry, vol 16. Elsevier, Amsterdam [Chapter 3]
Valeur B (2001) Molecular fluorescence: principles and applications. Weinheim, Wiley-VCH Verlag
Cossi M, Barone V, Cammi R, Tomasi J. Ab initio study of solvated mole-cules: a new implementation of the polarizable continuum model. Chem Phys Lett 1996; 255:327e35
Tomasi J, Mennucci B, Cammi R. Quantum mechanical continuum salvation models. Chem Rev 2005; 105:2999e3094.
Umape P; Gawale Y; Sekar N. J. Fluoresc. (in press) DOI 10.1007/s10895-014-1389-9
Bakhshiev NG (1964) Opt Spektrosk 16:821–832
Kawski A (1966) Acta Phys Pol 29:507–518
Chamma A, Viallet PCR (1970) Acad Sci Ser C 270:1901–1904
Kawski A (1964) Naturwissenschaften 51:82–83
Acknowledgments
The author is very much thankful to University Grant Commission (UGC), New Delhi, India for providing financial support and to IIT Mumbai for recording the 1H NMR, 13C NMR, and mass spectra.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1
(DOC 97 kb)
Figure S2
(DOC 87 kb)
Figure S3
(DOC 92 kb)
Figure S4
(DOC 73 kb)
Figure S5
(DOC 88 kb)
Figure S6
(DOC 81 kb)
Figure S7
(DOC 139 kb)
Figure S8
(DOC 150 kb)
Figure S9
(DOC 143 kb)
Figure S10
(DOC 146 kb)
Figure S11
(DOC 139 kb)
Figure S12
(DOC 135 kb)
Figure S13
(DOC 152 kb)
Figure S14
(DOC 89 kb)
Table S1
(DOC 30 kb)
Table S2
(DOC 34 kb)
Table S3
(DOC 46 kb)
Table S4
(DOC 38 kb)
Table S5
(DOC 38 kb)
Table S6
(DOC 38 kb)
Table S7
(DOC 38 kb)
Rights and permissions
About this article
Cite this article
Sekar, N., Umape, P.G., Kothavale, S. et al. Synthesis of Novel Carbazole based Styryl: Rational Approach for Photophysical Properties and TD-DFT. J Fluoresc 24, 1457–1472 (2014). https://doi.org/10.1007/s10895-014-1429-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-014-1429-5