Abstract
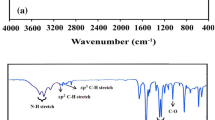
The sensitization of the excited triplet state of a novel symmetrical Bis(dialkylamino)phenoxazinium salt was developed in the presence of Hg2+. This effect was used to determine the concentration of Hg2+ in different water samples. The phenoxazinium salt sensor was characterized by different spectroscopic tools such as: UV, FTIR, NMR and fluorescence spectra. The sensor has an emission band at 347 nm in DMSO. Hg2+ in DMSO at pH 5.6 can remarkably quench the fluorescence intensity of the sensor at 347 nm and a new band was appeared at 436 nm due to the strong complex formation between Hg2+ and sensor. The quenching of the band intensity at 347 and the enhancement of the intensity of the new band at 436 were used to determine the Hg2+ in different waste water samples. The dynamic range found for the determination of Hg2+ concentration is 8.7 × 10-10 – 1.4 × 10-6 mol L−1 with a detection limit of 5.8 × 10−10 mol L−1 and quantification detection limit of 1.8 × 10-9 mol L-1.








Similar content being viewed by others
References
Lee JS, Han MS, Mirkin CA (2007) Angew Chem Int Ed 46:4093–4096
Boening DW (2000) Chemosphere 40:1335–1351
Onyido I, Norris AR, Buncel E (2004) Chem Rev 104:5911–5929
Tseng CM, Diego AD, Martin FM, Amouroux D, Donard OFX (1997) J Anal At Spectrom 12:743–750
Li YF, Chen CY, Li B, Sun J, Wang JX, Gao YX, Zhao YL, Chai ZF (2006) J Anal At Spectrom 21:94–96
Vallant B, Kadnar R, Goessler W (2007) J Anal At Spectrom 22:322–325
Chen YW, Tong J, D’Ulivo A, Belzile N (2002) Analyst 127:1541–1546
Anthemidis AN, Zachariadis GA, Michos CE, Stratis JA (2004) Anal Bioanal Chem 379:764–769
Pérez-Marín L, Otazo-Sánchez E, Macedo-Miranda G, Avila-Pérez P, Alonso Chamaro J, López-Valdivia H (2000) Analyst 125:1787–1790
Yantasee W, Lin YH, Zemanian TS, Fryxell GE (2003) Analyst 128:467–472
Caballero A, Lloveras V, Curiel D, Tárrage A, Espinosa A, Garcia R, Vidal-Gancedo J, Rovira C, Wurst K, Molina P, Veciana J (2007) Inorg Chem 46:825–838
Tan J, Yan XP (2008) Talanta 76:9–14
McClure DS (1952) J Chem Phys 20:682–686
Métivier R, Leray I, Valeur B (2004) Chem Eur J 10:4480–4490
Moon SY, Youn NJ, Park SM, Chang SK (2005) J Org Chem 70:2394–2397
Zhu XJ, Fu ST, Wong WK, Guo JP, Wong WY (2006) Angew Chem 45:3222–3226
Kim SH, Song KC, Ahn S, Kang YS, Chang SK (2006) Tetrahedron Lett 47:497–500
Yu Y, Lin LR, Yang KB, Zhong X, Huang RB, Zheng LS (2006) Talanta 69:103–106
Pandey S, Azam A, Pandey S, Chawla HM (2009) Org Biomol Chem 7:269–279
Nolan EM, Lippard SJ (2003) J Am Chem Soc 125:14270–14271
Nolan EM, Lippard SJ (2007) J Am Chem Soc 129:5910–5918
Zheng H, Qian ZH, Xu L, Yuan FF, Lan LD, Xu JG (2006) Org Lett 8:859–861
Shi W, Ma HM (2008) Chem Commun 16:1856–1858
Huang JH, Xu YF, Qian XH (2009) J Org Chem 74:2167–2170
Liu W, Xu LW, Zhang HY, You JJ, Zhang XL, Sheng RL, Li H, Wu S, Wang PF (2009) Org Biomol Chem 7:660–664
Essawy AA, Attia MS (2013) Talanta 107:18–24
Attia MS, Essawy AA, Youssef AO (2012) Anal Methods. 8:2323–2328
Attia MS, Essawy AA, Youssef AO, Abdel-Mottaleb MSA (2012) J Luminesc 132(10):2741–2746
Attia MS, Mahmoud WH, Ramsis MN, Khalil LH, Othman AM, Hashem SG, Mostafa MS (2011) J Fluoresc 21(4):1739–1748
Attia MS, Othman AM, El-Raghi E, Aboul-Enein HY (2011) J Fluoresc 21(2):739–745
Miller JC, Miller JN (1994) Statistics for analytical chemistry, 4th edn. Ellis-Howood, New York, p 115
Guptaa VK, Chandrab S, Langb H (2005) Talanta 66:575–580
Fang Z, Liu B (2008) Tetrahedron Lett 49:2311–2315
Yari A, Papi F (2009) Sensors Actuators B 138:467–473
Chen J, Gao Y, Xu Z, Wu G, Chen Y, Zhu C (2006) Anal Chim Acta 577:77–84
Shamsipur M, Hosseini M, Alizadeh K, Alizadeh N, Yari A, Caltagirone C, Lippolis V (2005) Anal Chim Acta 533:17–24
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Attia, M.S., Youssef, A.O., Elgazwy, AS.S.H. et al. Synthesis and Characterization of New Light Emitter Symmetrical Phenoxazinium Salt and Its Potential Application as Sensor for Assessment of Hg2+ . J Fluoresc 24, 759–765 (2014). https://doi.org/10.1007/s10895-014-1349-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-014-1349-4