Abstract
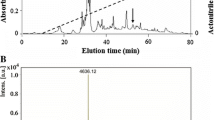
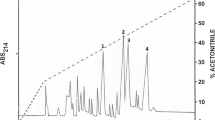
Population declines due to amphibian chytridiomycosis among selected species of ranid frogs from western North America have been severe, but there is evidence that the Oregon spotted frog, Rana pretiosa Baird and Girard, 1853, displays resistance to the disease. Norepinephrine-stimulated skin secretions were collected from a non-declining population of R. pretiosa that had been exposed to the causative agent Batrachochytrium dendrobatidis. Peptidomic analysis led to identification and isolation, in pure form, of a total of 18 host-defense peptides that were characterized structurally. Brevinin-1PRa, -1PRb, -1PRc, and -1PRd, esculentin-2PRa and -PRb, ranatuerin-2PRa, -2PRb, -2PRc, and -2PRe, temporin-PRb and -PRc were identified in an earlier study of skin secretions of frogs from a different population of R. pretiosa known to be declining. Ranatuerin-2PRf, -2PRg, -2PRh, temporin-PRd, -PRe, and -PRf were not identified in skin secretions from frogs from the declining population, whereas temporin-PRa and ranatuerin-2PRd, present in skin secretions from the declining population, were not detected in the current study. All purified peptides inhibited the growth of B. dendrobatidis zoospores. Peptides of the brevinin-1 and esculentin-2 families displayed the highest potency (minimum inhibitory concentration = 6.25–12.5 μM). The study provides support for the hypothesis that the multiplicity and diversity of the antimicrobial peptide repertoire in R. pretiosa and the high growth-inhibitory potency of certain peptides against B. dendrobatidis are important in conferring a measure of resistance to fatal chytridiomycosis.




Similar content being viewed by others
References
Briggs CJ, Knapp RA, Vredenburg VT (2010) Enzootic and epizootic dynamics of the chytrid fungal pathogen of amphibians. Proc Natl Acad Sci U S A 107:9695–9700
Clinical laboratory and Standards Institute (2008) Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved Standard M07-A8. CLSI, Wayne, PA
Conlon JM (2008) Reflections on a systematic nomenclature for antimicrobial peptides from the skins of frogs of the family Ranidae. Peptides 29:1815–1819
Conlon JM (2011) The contribution of skin antimicrobial peptides to the system of innate immunity in anurans. Cell Tissue Res 343:201–212
Conlon JM, Sonnevend A, Patel M, Davidson C, Nielsen PF, Pal T, Rollins-Smith LA (2003) Isolation of peptides of the brevinin-1 family with potent candidacidal activity from the skin secretions of the frog Rana boylii. J Pept Res 62:207–213
Conlon JM, Kolodziejek J, Nowotny N (2009) Antimicrobial peptides from the skins of North American frogs. Biochim Biophys Acta 1788:1556–1563
Conlon JM, Mechkarska M, Ahmed E, Coquet L, Jouenne T, Leprince, Vaudry H, Hayes MP, Padgett-Flohr GJ (2011) Host defense peptides in skin secretions of the Oregon spotted frog Rana pretiosa: implications for species resistance to chytridiomycosis. Dev Comp Immunol 35:644–649
Davidson C, Benard MF, Shaffer HB, Parker JM, O’Leary C, Conlon JM, Rollins-Smith LA (2007) Effects of chytrid and carbaryl exposure on survival, growth and skin peptide defenses in foothill yellow-legged frogs. Environ Sci Technol 41:1771–1776
Fisher MC, Garner TW, Walker SF (2009) Global emergence of Batrachochytrium dendrobatidis and amphibian chytridiomycosis in space, time, and host. Annu Rev Microbiol 63:291–310
Gammill WM, Fites JS, Rollins-Smith LA (2012) Norepinephrine depletion of antimicrobial peptides from the skin glands of Xenopus laevis. Dev Comp Immunol 37:19–27
Greenspan SE, Calhoun AJ, Longcore JE, Levy MG (2012) Transmission of Batrachochytrium dendrobatidis to wood frogs (Lithobates sylvaticus) via a bullfrog (L. catesbeianus) vector. J Wildl Dis 48:575–582
Hammerson G, Pearl C (2012) Rana pretiosa. In: International Union for Conservation of Nature 2012. IUCN Red List of Threatened Species. Version 2012.1. Electronic database accessible at www.iucnredlist.org
James TY, Litvintseva AP, Vilgalys R, Morgan JA, Taylor JW, Fisher MC, Berger L, Weldon C, Du Preez L, Longcore JE (2009) Rapid global expansion of the fungal disease chytridiomycosis into declining and healthy amphibian populations. PLoS Pathog 5:e1000458
Kaiser K, Pollinger J (2012) Batrachochytrium dendrobatidis shows high genetic diversity and ecological niche specificity among haplotypes in the Maya Mountains of Belize. PLoS One 7:e32113
Longcore JE, Pessier AP, Nichols DK (1999) Batrachochytrium dendrobatidis gen.et sp. Nov., a chytrid pathogenic to amphibians. Mycologia 91:219–227
Matutte B, Storey KB, Knoop FC, Conlon JM (2000) Induction of synthesis of an antimicrobial peptide in the skin of the freeze-tolerant frog, Rana sylvatica, in response to environmental stimuli. FEBS Lett 483:35–138
Nishida M, Imura Y, Yamamoto M, Kobayashi S, Yano Y, Matsuzaki K (2007) Interaction of a magainin-PGLa hybrid peptide with membranes: insight into the mechanism of synergism. Biochemistry 46:14284–14290
Padgett-Flohr GE (2008) Pathogenicity of Batrachochytrium dendrobatidis in two threatened California amphibians. Herpetol Conserv Biol 3:182–191
Padgett-Flohr GE, Hayes MP (2011) Assessment of the vulnerability of the Oregon spotted frog (Rana pretiosa) to the amphibian chytrid fungus (Batrachochytrium dendrobatidis). Herpetol Conserv Biol 6:99–106
Pask JD, Woodhams DC, Rollins-Smith LA (2012) The ebb and flow of antimicrobial skin peptides defends northern leopard frogs (Rana pipiens) against chytridiomycosis. Glob Chang Biol 18:1231–1238
Pearl CA, Hayes MP (2005) Rana pretiosa, Oregon spotted frog. In: Lannoo MJ (ed) Amphibian declines: The conservation status of United States species. University of California Press, Berkeley, CA, pp 577–580
Pearl CA, Bowerman J, Adams MJ, Chelgren ND (2009) Widespread occurrence of the chytrid fungus Batrachochytrium dendrobatidis on Oregon Spotted Frogs (Rana pretiosa). EcoHealth 6:209–218
Rachowicz LJ, Vredenburg VT (2004) Transmission of Batrachochytrium dendrobatidis within and between amphibian life stages. Dis Aquat Org 61:75–83
Ramsey JP, Reinert LK, Harper LK, Woodhams DC, Rollins-Smith LA (2010) Immune defenses against Batrachochytrium dendrobatidis, a fungus linked to global amphibian declines, in the South African clawed frog, Xenopus laevis. Infect Immun 78:3981–3992
Rollins-Smith LA (2009) The role of amphibian antimicrobial peptides in protection of amphibians from pathogens linked to global amphibian declines. Biochim Biophys Acta 1788:1593–1599
Rollins-Smith LA, Carey C, Longcore J, Doersam JK, Boutte A, Bruzgal JE, Conlon JM (2002) Activity of antimicrobial skin peptides from ranid frogs against Batrachochytrium dendrobatidis, the chytrid fungus associated with global amphibian declines. Dev Comp Immunol 26:471–479
Rollins-Smith LA, Ramsey JP, Reinert LK, Woodhams DC, Livo LJ, Carey C (2009) Immune defenses of Xenopus laevis against Batrachochytrium dendrobatidis. Front Biosci (Sch Ed) 1:68–91
Rosenblum EB, Poorten TJ, Settles M, Murdoch GK (2012) Only skin deep: shared genetic response to the deadly chytrid fungus in susceptible frog species. Mol Ecol 21:3110–3120
Tennessen JA, Woodhams DC, Chaurand P, Reinert LK, Billheimer D, Shyr Y, Caprioli RM, Blouin MS, Rollins-Smith LA (2009) Variations in the expressed antimicrobial peptide repertoire of northern leopard frog (Rana pipiens) populations suggest intraspecies differences in resistance to pathogens. Dev Comp Immunol 33:1247–1257
Tobler U, Borgula A, Schmidt BR (2012) Populations of a susceptible amphibian species can grow despite the presence of a pathogenic chytrid fungus. PLoS One 7:e34667
Voordouw MJ, Adama D, Houston B, Govindarajulu P, Robinson J (2010) Prevalence of the pathogenic chytrid fungus, Batrachochytrium dendrobatidis, in an endangered population of northern leopard frogs. Rana pipiens. BMC Ecol 10:6
Voyles J, Vredenburg VT, Tunstall TS, Parker JM, Briggs CJ, Rosenblum EB (2012) Pathophysiology in mountain yellow-legged frogs (Rana muscosa) during a chytridiomycosis outbreak. PLoS One 7:e35374
Acknowledgments
This work was supported by a Faculty Support Grant and a University/National Research Foundation Grant from U.A.E. University to J.M.C. and NSF grant 1121758 to L.R-S. The authors thank Marko Anderson of the Washington Department of Corrections and Carri LeRoy and Kelli Bush of the Sustainability in Prisons Project, and Allison Abrahamse and Rich Sartor of Northwest Trek, for help in collecting skin secretions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Conlon, J.M., Reinert, L.K., Mechkarska, M. et al. Evaluation of the Skin Peptide Defenses of the Oregon Spotted Frog Rana pretiosa Against Infection by the Chytrid Fungus Batrachochytrium dendrobatidis . J Chem Ecol 39, 797–805 (2013). https://doi.org/10.1007/s10886-013-0294-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-013-0294-z