Abstract
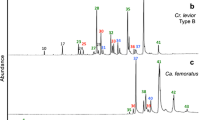
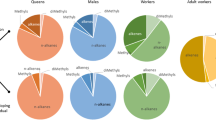
Neotropical attine ants live in obligatory symbiosis with a fungus that they grow for food on a substrate of primarily plant material harvested by workers. Nestmate recognition is likely based on chemical cues as in most other social insects, but recent studies have indicated that both the ants and their mutualistic fungi may contribute to the recognition templates. To investigate the within-colony variation in chemical profiles, we extracted and identified compounds from the cuticle of workers, the postpharyngeal gland of workers, ant pupae and larvae, and the fungal symbiont of three species of higher attine ants: Atta colombica, Acromyrmex echinatior, and Sericomyrmex amabilis. The relative proportions of identified compounds were compared and represented 11 classes: n-alkanes, alkenes, branched methylalkanes, branched dimethylalkanes, trimethylalkanes, branched alkenes, aldehydes, alcohols, acetates, acids, and esters. The chemical profiles in all three species are likely to be sufficiently different to allow discrimination at the species and colony level and sufficiently similar within colonies to generate a relatively constant colony-specific chemical gestalt. The relative likelihood of individual compounds being derived from the ants, the ant brood, or the fungal symbiont are discussed. We hypothesize that hydrocarbons are particularly important as recognition cues because they appear to simultaneously allow the assessment of developmental stages and the identification of symbiont, colony, and species.


Similar content being viewed by others
References
Akino, T. and Yamaoka, R. 1998. Chemical mimicry in the root aphid parasitoid Paralipsis eikoae Yasumatsu (Hymenoptera: aphidiidae) of the aphid attending ant Lasius sakagamii Yamauchi and Hayashida (Hymenoptera: Formicidae). Chemoecology 8:153–161.
Beye, M., Neumann, P., and Moritz, R. F. A. 1997. Nestmate recognition and the genetic gestalt in the mound-building ant Formica polyctena. Insectes Soc. 44:49–58.
Beye, M., Neumann, P., Chapuisat, M., and Pamilo, P., and Moritz, R. F. A. 1998. Nestmate recognition and the genetic relatedness of nests in the ant Formica pratensis. Behav. Ecol. Sociobiol. 43:67–72.
Blomquist, G. J., Howard, R. W., and McDaniel, C. A. 1979. Structures of the cuticular hydrocarbons of the termites Zootermopsis angusticollis (Hagen). Insect Biochem. 9:365–370.
Bonavita-Cougourdan, A., Clément, J., and Lange, C. 1989. The role of cuticular hydrocarbons in the recognition of larvae by workers of the ant Camponotus vagus: changes in the chemical signature in response to social environment (Hymenoptera: Formicidae). Sociobiology 16:49–74.
Boomsma, J. J., Nielsen, J., Sundström, L., Oldham, N. J., Tentschert, J., Petersen, H. C., and Morgan, E. D. 2003. Informational contraints on optimal sex allocation in ant. Proc. Natl. Acad. Sci. USA 100:158799–8804.
Bot, A. N. M., Rehner, S. A., and Boomsma, J. J. 2001. Partial incompatibility between ants and symbiotic fungi in two sympatric species of Acromyrmex leaf-cutting ants. Evolution 55:1980–1991.
Brandão, C. R. F. and Mayhé-Nunes, A. J. 2001. A new fungus-growing genus, Mycetagroicus gen. n., with the description of three new species and comments on the monophyly of the Attini (Hymenoptera: Formicidae). Sociobiology 38:639–665.
Breed, M. D. 1983. Nestmate recognition in honey bees. Anim. Behav. 31:86–91.
Carlson, D. A., Bernier, U. R., and Sutton, B. C. 1998. Evolution patterns from capillary GC for methyl-branched alkanes. J. Chem. Ecol. 24:1845–1865.
Chapela, I. H., Rehner, S. A., Schultz, T. R., and Mueller, U. G. 1994. Evolutionary history of the symbiosis between fungus-growing ants and their fungi. Science 266:1691–1694.
Crosland, M. W. J. 1989. Kin recognition in the ant Rhytidoponera confusa. I. Environmental odour. Anim. Behav. 37:912–919.
Currie, C. R., Wong, B., Stuart, A. E., Schultz, T. R., Rehner, S. A., Mueller, U. G., Sung, G. H., Spatafora, J. W., and Straus, N. A. 2003. Ancient tripartite coevolution in the Attine ant–microbe symbiosis. Science 299:386–388.
Currie, C. R., Poulsen, M., Mendenhall, J., Boomsma, J. J., and Billen, J. 2006. Coevolved crypts and exocrine glands support mutualistic bacteria in fungus-growing ants. Science 311:81–83.
Elmes, G. W., Akino, T., Thomas, J. A., Clarke, R. T., and Knapp, J. J. 2002. Interspecific differences in cuticular hydrocarbon profiles of Myrmica ants are sufficiently consistent to explain host specificity by Maculinea (large blue) butterflies. Oecologia 130:525–535.
Heinze, J., Foitzik, S., Hippert, A., and Hölldobler, B. 1996. Apparent dear-enemy phenomenon and environment-based recognition cues in the ant Leptothorax nylanderi. Ethology 102:510–522.
Heinze, J., Stengl, B., and Sledge, M. F. 2002. Worker rank, reproductive status and cuticular hydrocarbon signature in the ant, Pachycondyla cf. inversa. Behav. Ecol. Sociobiol. 52:59–65.
Hinkle, G., Wetterer, J. K., Schultz, T. R., and Sogin, M. L. 1994. Phylogeny of the attine ant fungi based on analysis of small subunit ribosomal RNA gene sequences. Science 266:1695–1697.
Hölldobler, B. and Wilson, E. O. 1990. The ants. 732 pp. Belknap, Cambridge, MA.
Howard, R. W. and Blomquist, G. J. 2005. Ecological, behavioral, and biochemical aspects of insect hydrocabons. Annu. Rev. Entomol. 50:371–393.
Howard, R. W., Mcdaniel, C. A., and Blomquist, G. J. 1978. Cuticular hydrocarbons of the eastern subterranean termite, Reticulitermes flavipes (Kollar) (Isoptera: Rhinotermitidae). J. Chem. Ecol. 4:233–245.
Howard, R. W., Mcdaniel, C. A., Nelson, D. R., Blomquist, G. J., Gelbaum, L. T., and Zalkow, L. H. 1982. Cuticular hydrocarbons of Reticulitermes virginicus (BANKS) and their role as potential species and caste-recognition cues. J. Chem. Ecol. 8:1227–1239.
Jutsum, A. R., Saunders, T. S., and Cherrett, J. M. 1979. Intraspecific aggression in the leaf-cutting ant Acromyrmex octospinosus. Anim. Behav. 27:839–844.
Lahav, S., Soroker, V., Hefetz, A., and Vander Meer, R. K. 1999. Direct behavioral evidence for hydrocarbons as ant recognition discriminators. Naturwissenschaften 86:246–249.
Lambardi, D., Dani, F. R., Turillazzi, S., and Boomsma, J. 2007. Incipient social parasites of Acromyrmex leaf-cutting ants avoid host aggression by being chemically inconspicuous. Behav. Ecol. Sociobiol. 61:843–851.
Liang, D. and Silverman, J. 2000. You are what you eat: diet modifies cuticular hydrocarbons and nestmate recognition in the Argentine ant, Linepithema humile. Naturwissenschaften 87:412–416.
Lommelen, E., Johnson, C. A., Drijfhout, F. P., Billen, J., Wenseleers, T., and Gobin, B. 2006. Cuticular hydrocarbons provide reliable cues of fertility in the ant Gnamptogenys striatula. J. Chem. Ecol. 32:2023–2034.
Mikheyev, A. S., Mueller, U. G., and Boomsma, J. J. 2007. Population genetic signatures of diffuse co-evolution between leaf-cutting ants and their cultivar fungi. Mol. Ecol. 16:209–216.
Mueller, U. G. 2002. Ant versus fungus versus mutualism: ant cultivar conflict and the deconstruction of the Attine ant–fungus symbiosis. Am. Nat. 160:S67–S98.
Mueller, U. G., Rehner, S. A., and Schultz, T. R. 1998. The evolution of agriculture in ants. Science 281:2034–2038.
Mueller, U. G., Schultz, T. R., Currie, C. R., Adams, R. M. M., and Malloch, D. 2001. The origin of the Attine ant–fungus mutualism. Q. Rev. Biol. 76:169–197.
Nielsen, J., Boomsma, J. J., Oldham, N. J., Petersen, H. C., and Morgan, E. D. 1999. Colony-level and season-specific variation in cuticular hydrocarbon profiles of individual workers in the ant Formica truncorum. Insect. Soc. 46:58–65.
Obin, M. S. 1986. Nestmate recognition cues in laboratory and field colonies of Solenopsis invicta Buren (Hymenoptera: Formicidae): effect of environment and the role of cuticular hydrocarbons. J. Chem. Ecol. 12:1965–1975.
Poulsen, M. and Boomsma, J. J. 2005. Mutualistic fungi control crop diversity in fungus-growing ants. Science 307:741–744.
Richard, F. J., Hefetz, A., Christides, J. P., and Errard, C. 2004. Food influence on colonial recognition and chemical signature between nestmates in the fungus-growing ant Acromyrmex subterraneus. Chemoecology 14:9–16.
Richard, F. J., Mora, P., Errard, C., and Rouland, C. 2005. Digestive capacities of leaf-cutting ants and the contribution of their fungal cultivar to the degradation of plant material. J. Comp. Physiol. B 175:297–303.
Richard, F. J., Poulsen, M., Hefetz, A., Errard, C., Nash, D. R., and Boomsma, J. J. 2007. The origin of chemical profiles of fungal symbionts and their significance for nestmate recognition in Acromyrmex leaf-cutting ants. Behav. Ecol. Sociobiol. 61:1637–1649.
Schultz, T. R. and Meier, R. 1995. A phylogenetic analysis of the fungus-growing ants (Hymenoptera: Formicidae: attini) based on morphological characters of the larvae. Syst. Entomol. 20:337–370.
Silverman, J. and Liang, D. 2001. Colony disassociation following diet partitioning in a unicolonial ant. Naturwissenschaften 88:73–77.
Singer, T. L. 1998. Roles of hydrocarbons in the recognition systems of insects. Am. Zool. 38:394–405.
Singer, T. L. and Espelie, K. E. 1996. Nest surface hydrocarbons facilitate nestmate recognition for the social wasp, Polistes metricus Say (Hymenoptera, Vespidae). J. Insect Behav. 9:857–870.
Soroker, V., Vienne, C., Hefetz, A., and Nowbahari, E. 1994. The postpharyngeal gland as a “Gestalt” organ for nestmate recognition in the ant Cataglyphis niger. Naturwissenschaften 81:510–513.
Soroker, V., Vienne, C., and Hefetz, A. 1995. Hydrocarbon dynamics within and between nestmates in Cataglyphis niger (Hymenoptera: Formicidae). J. Chem. Ecol. 21(3):365–378.
Vander Meer, R. K. and Morel, L. 1998. Nestmate recognition in ants, pp. 79–103, in R. K. Vander Meer, M. Breed, K. E. Espelie, M. Winston, (eds.). Pheromone Communication in Social Insects. Westview, Boulder, CO.
Viana, A. M. M., Frézard, A., Malosse, C., Della Lucia, T. M. C., Errard, C., and Lenoir, A. 2001. Colonial recognition of fungus in the fungus-growing ant Acromyrmex subterraneus subterraneus (Hymenoptera: Formicidae). Chemoecology 11:29–36.
Wagner, D., Tissot, M., Cuevas, W., and Gordon, D. M. 2000. Harvester ants utilize cuticular hydrocarbons in nestmate recognition. J. Chem. Ecol 26:2245–2257.
Weber, N. A. 1972. Gardening ants, the attines. Memoirs of the American Philosophical Society, Philadelphiap xvii + 146.
Wilson, E. O. 1971. The insect societies. 548 pp. Belknap, Cambridge, MA.
Acknowledgements
We thank the Smithsonian Tropical Research Institute (STRI) for providing logistic help and facilities to work in Gamboa and the Autoridad Nacional del Ambiente y el Mar (ANAM) for permission to sample ant colonies in Panama and export them to Denmark. Fieldwork was supported by grants from the Carlsberg Foundation and the Danish Natural Science Research Council to JJB. All experiments performed in this manuscript comply with current Danish and USA law.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Richard, FJ., Poulsen, M., Drijfhout, F. et al. Specificity in Chemical Profiles of Workers, Brood and Mutualistic Fungi in Atta, Acromyrmex, and Sericomyrmex Fungus-growing Ants. J Chem Ecol 33, 2281–2292 (2007). https://doi.org/10.1007/s10886-007-9385-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-007-9385-z