Abstract
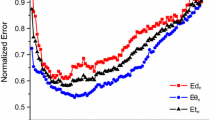
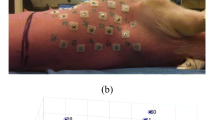
To improve spatial resolution in recordings of intra-cardiac electrograms we characterized the utility of a novel configuration of two recording electrodes arranged perpendicularly to the endocardial surface. We hypothesized that this configuration denoted as orthogonal close unipolar (OCU) would combine advantages of conventional unipolar and contact bipolar (CBP) configurations. Electrical excitation was simulated in a computational model as arising from dipole current or from multi-wavelet reentry sources. Recordings were calculated for electrode tips 1 mm above the plane of the heart. Analogous recordings were obtained from swine hearts. Electrograms recorded with CBP showed strong dependence on orientation of the electrode pair with respect to the direction of spread of tissue excitation. By contrast, OCU recordings exhibited no directional dependence. OCU was significantly superior to CBP with respect to avoidance of far-field confounding of local tissue activity; the average far-field/near-field ratios for CBP and OCU were 0.09 and 0.05, respectively, for the simulated dipole current sources. In the swine hearts the ratios of ventricular to atrial signals for CBP and OCU were 0.15 ± 0.07 and 0.08 ± 0.09, respectively (p < 0.001). The difference between the actual dominant frequency in the tissue and that recorded by the electrodes was 0.44 ± 0.33 Hz for OCU, 0.58 ± 0.40 Hz for unipolar, and 0.62 ± 0.46 Hz for CBP. OCU confers improved spatial resolution compared with both unipolar and CBP electrode configurations. Unlike the case with CBP, OCU recordings are independent of excitation wave-front direction.







Similar content being viewed by others
References
Berbari EJ, Dyer J, Lander P, Geselowitz DB. Simulation of intracardiac electrograms with a moving dipole source: role of electrode geometry and high pass filtering. J Electrocardiol. 1994;27(Suppl):146–50.
Stinnett-Donnelly JM, Thompson N, Habel N, Petrov-Kondratov V, de Correa Sa DD, Bates JH, Spector PS. Effects of electrode size and spacing on the resolution of intracardiac electrograms. Coron Artery Dis. 2012;23:126–32.
DeCaprio V, Hurzeler P, Furman S. A comparison of unipolar and bipolar electrograms for cardiac pacemaker sensing. Circulation. 1977;56:750–5.
Kimber S, Downar E, Masse S, Sevaptsidis E, Chen T, Mickleborough L, Parsons I. A comparison of unipolar and bipolar electrodes during cardiac mapping studies. Pacing Clin Electrophysiol. 1996;19:1196–204.
Plonsey R, Barr RC. Bioelectricity: a quantitative approach (3rd ed). New York: Springer; 2007.
Spector PS, Habel N, Sobel BE, Bates JH. Emergence of complex behavior: an interactive model of cardiac excitation provides a powerful tool for understanding electric propagation. Circ Arrythm Electrophysiol. 2011;4:586–91.
Nademanee K, McKenzie J, Kosar E, Schwab M, Sunsaneewitayakul B, Vasavakul T, Khunnawat C, Ngarmukos T. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol. 2004;43:2044–53.
Umapathy K, Masse S, Kolodziejska K, Veenhuyzen GD, Chauhan VS, Husain M, Farid T, Downar E, Sevaptsidis E, Nanthakumar K. Electrogram fractionation in murine HL-1 atrial monolayer model. Heart Rhythm. 2008;5:1029–35.
Zlochiver S, Yamazaki M, Kalifa J, Berenfeld O. Rotor meandering contributes to irregularity in electrograms during atrial fibrillation. Heart Rhythm. 2008;5:846–54.
Kalifa J, Tanaka K, Zaitsev AV, Warren M, Vaidyanathan R, Auerbach D, Pandit S, Vikstrom KL, Ploutz-Snyder R, Talkachou A, Atienza F, Guiraudon G, Jalife J, Berenfeld O. Mechanisms of wave fractionation at boundaries of high-frequency excitation in the posterior left atrium of the isolated sheep heart during atrial fibrillation. Circulation. 2006;113:626–33.
Berenfeld O, Zaitsev AV, Mironov SF, Pertsov AM, Jalife J. Frequency-dependent breakdown of wave propagation into fibrillatory conduction across the pectinate muscle network in the isolated sheep right atrium. Circ Res. 2002;90:1173–80.
Gardner PI, Ursell PC, Fenoglio JJ Jr, Wit AL. Electrophysiologic and anatomic basis for fractionated electrograms recorded from healed myocardial infarcts. Circulation. 1985;72:596–611.
Lesh MD, Spear JF, Simson MB. A computer model of the electrogram: what causes fractionation? J Electrocardiol 21(Suppl):S69–73.
de Correa Sa DD, Thompson N, Stinnett-Donnelly J, Znojkiewicz P, Habel N, Müller JG, Bates JH, Buzas JS, Spector PS. Electrogram fractionation: the relationship between spatiotemporal variation of tissue excitation and electrode spatial resolution. Circ Arrythm Electrophysiol. 2011;4:909–16.
Benson BE, Carrick R, Habel N, Bielau P, Bates O, Spector P (2013) Mapping atrial fibrillation: high resolution electrograms identify circuit density. Poster #Computational Engineering-P-Fri-A-5 presented 09/27/2013 at the 2013 Biomedical Engineering Society Annual Meeting. 2013 Program Book available at http://bmes.org/agenda.
Habel N, Znojkiewicz P, Thompson N, Müller JG, Mason B, Calame J, Calame S, Sharma S, Mirchandani G, Janks D, Bates J, Noori A, Karnbach A, Lustgarten DL, Sobel BE, Spector P. The temporal variability of dominant frequency and complex fractionated atrial electrograms constrains the validity of sequential mapping in human atrial fibrillation. Heart Rhythm. 2010;7:586–93.
Acknowledgments
This work was supported in part by a grant from Medtronic, Inc.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thompson, N.C., Stinnett-Donnelly, J., Habel, N. et al. Improved spatial resolution and electrogram wave direction independence with the use of an orthogonal electrode configuration. J Clin Monit Comput 28, 157–163 (2014). https://doi.org/10.1007/s10877-013-9508-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-013-9508-8