Abstract
Adsorption and dissociation of H2 on cluster Al6N are investigated based on density functional theory with generalized gradient approximation. The stable geometrical structures of both reactants and products, as well as the transition states and reaction paths for dissociation, are investigated. Results show that H2@Al6N has low adsorption energies, implying that they are unsuitable for direct storage of H2. However, the energy barrier for each reaction is found within 0.6226–1.1256 eV, implicating that Al6N can store hydrogen through dissociation of H2 under appropriate conditions.
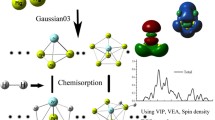
Graphical Abstract
Adsorption and dissociation of H2 on Al6N cluster are investigated. The energy barrier for each reaction is found within 0.6226–1.1256 eV, implicating that Al6N can store hydrogen through dissociation of H2 under appropriate conditions.








Similar content being viewed by others
References
N. S. Lewis and D. G. Nocera (2006). Natl. Acad. Sci. 103, 15729.
D. G. Nocera (2006). Daedalus 135, 112.
R. Eisenberg and D. G. Nocera (2005). Inorg. Chem. 44, 6799.
A. J. Esswein and D. G. Nocera (2007). Chem. Eng. News 107, 4022.
J. Moc, D. G. Musaev, and K. Morokuma (2000). J. Phys. Chem. A 104, 11606.
Q. Cui, D. G. Musaev, and K. Morokuma (1998). J. Chem. Phys. 108, 8418.
J. Moc, D. G. Musaev, and K. Morokuma (2003). J. Phys. Chem. A 107, 4929.
C. Zhou, S. Yao, and J. Wu (2008). Phys. Chem. Chem. Phys. 10, 5445.
C. Zhou, S. Yao, and J. Wu (2009). J. Comput. Theor. Nanosci. 6, 1320.
Y. Zhao and D. Tian (2012). Comput. Theor. Chem. 991, 40.
G. Kresse and J. Furthmüller (1996). Phys. Rev. B 54, 11169.
G. Kresse and J. Furthmüller (1996). Comput. Mater. Sci. 6, 15.
X. J. Liu, D. X. Tian, and C. G. Meng (2015). J. Mol. Struct. 1080, 105.
R. Jin, S. L. Zhang, Y. H. Zhang, and S. P. Huang (2011). Int. J. Hydrogen Energy 36, 9069.
I. Pino, G. J. Kroes, and M. C. Van Hemert (2010). J. Chem. Phys. 133, 184304.
H. Yang, Y. Zhang, and H. S. Chen (2014). J. Chem. Phys. 141, 064302.
K. N. Li, C. L. Yang, M. S. Wang, X. G. Ma, and L. Z. Wang (2015). Int. J. Hydrogen Energy 40, 8911.
A. D. Becke (1993). J. Chem. Phys. 98, 5648.
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, and J. R. Cheeseman Gaussian 09, Revision D.01 (Gaussian, Inc, Wallingford CT, 2013).
J. Sun, W. C. Lu, H. Wang, Z. S. Li, and C. C. Sun (2006). J. Phys. Chem. A 110, 2729.
D. M. Cox, D. J. Trevor, R. L. Whetten, and A. Kaldor (1988). J. Phys. Chem. 92, 421.
L. Guo and Y. F. Yang (2013). Int. J. Hydrogen Energy 38, 3640.
Acknowledgement
This work was supported by the National Natural Science Foundation of China (NSFC) (Grant Nos. NSFC-11574125 and NSFC-11374132) and the Taishan Scholar Project of Shandong Province (ts201511055).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, KN., Yang, CL., Wang, MS. et al. Adsorption and Dissociation of H2 on Cluster Al6N. J Clust Sci 28, 1335–1344 (2017). https://doi.org/10.1007/s10876-016-1151-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-016-1151-3