Abstract
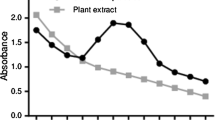
The development of phytomediated nanoparticles synthesis is receiving increasing attention due to ease of preparation, less chemical handling, and eco-friendly. In the present study, crystallization of silver ions to nanosized particles by aqueous leaves extract of Cassia javanica through bioreduction process was assessed. Strong plasmon resonance of silver nanoparticles was observed around 435 nm. UV–Vis spectroscopy, transmission electron microscope and Fourier transform infrared spectroscopy were performed to examine the formation of silver nanoparticles (AgNPs). The antibacterial activity of AgNPs was tested against four pathogenic Gram-negative bacteria. AgNPs showed strong antibacterial activity. The strongest activity was recorded on Pseudomonas aeruginosa with 34 mm zone of inhibition followed by Escherichia coli (28 mm), Enterobacter aerogenes (25 mm) and Salmonella typhimurium (23 mm) respectively. The Gram-negative bacteria were highly sensitive to AgNPs, whereas less sensitive to silver nitrate (AgNO3) and C. javanica leaves extract. The AgNPs were also evaluated for the estimation of total phenolic content. It is concluded that leaves extracts can be used for the synthesis of AgNPs that is environmentally friendly and cost effective. These preparations can be used for various biotechnology and medical applications for controlling pathogenic bacteria.






Similar content being viewed by others
References
H. W. Boucher, G. H. Talbot, and J. S. Bradley (2009). Clin. Infect. Dis. 48, 1–12.
L. B. Rice (2008). J. Infect. Dis. 197, 1079–1081.
T. N. V. K. V. Prasad and E. K. Elumalai (2011). Asian Pac. J. Trop. Biomed. 1, 439–442.
C. Krishnaraj, P. Muthukumaran, R. Ramachandran, M. D. Balakumaran, and P. T. Kalaichelvan (2014). Biotechnol. Rep. 4, 42–49.
I. Sondi and S. B. Sondi (2004). J. Colloid Interface Sci. 275, 177–182.
G. Zhao and J. Stevens (1998). Biometals 11, 27–32.
S. Pavagadhi, M. Sathishkumar, and R. Balasubramanian (2014). Water Res. 55, 280–291.
A. R. Binupriya, M. Sathishkumar, and Y. Soon (2010). Ind. Eng. Chem. Res. 49, 852–858.
S. Prabhu and E. K. Poulose (2012). Int. Nano. Lett. 2, 32–42.
R. S. Suganya, K. B. Priya, and S. Roxy (2012). IRJP 3, 285–288.
T. J. Beveridge, M. N. Hughes, H. Lee, K. T. Leung, R. K. Poole, I. Savvaidis, S. Silver, and J. T. Trevors (1997). Adv. Microb. Physiol. 38, 178.
M. I. Sriram, K. Kalishwaralal, and S. Gurunathan (2012). Methods Mol. Biol. 906, 33–43.
G. Li, D. He, Y. Qian, B. Guan, Y. Cui, S. Gao, K. Yokoyama, and L. Wang (2012). Int. J. Mol. Sci. 13, 466–476.
T. Elavazhagan and K. D. Arunachalam (2011). Int. J. Nanomed. 6, 1265–1278.
D. M. Ali, N. Thajuddin, K. Jeganathan, and M. Gunasekaran (2011). Colloids Surf. B 85, 360–365.
V. Kumar and S. K. Yadav (2009). J. Chem. Technol. Biotechnol. 84, 151–157.
K. B. Narayanan and N. Sakthivel (2008). Coriander leaf mediated biosynthesis of gold nanoparticles. Mater. Lett. 62, 4588–4590.
P. Venkatachalam, R. Kalaiarasi, and N. Jayalakshmi (2010). Plant Cell Biotechnol. Mol. Biol. 11, 1–16.
K. P. Chittam and S. L. Deore (2013). J. Biomed. Pharm. Res. 2, (1), 33–35.
U. C. Kumavat, S. N. Shimpi, and S. P. Jagdale (2012). J. Adv. Pharm. Tech. Res. 3, (1), 47–51.
P. Kaur and S. Arora (2008). J. China Clin. Med. 5, (8), 457–462.
S. Ganesan (2008). Natl. Prod. Rad. 7, (2), 166–172.
H. Y. Cheng, C. M. Yang, T. C. Lin, D. E. Shieh, and C. C. Lin (2006). J. Med. Microbiol. 55, 201–206.
J. I. Pandith (2012). J. Drug Deliv. Ther. 2, (4), 135–138.
R. W. Bauer, M. D. K. Kirby, J. C. Sherris, and M. Turck (1966). Am. J. Clin. Pathol. 45, 493–496.
C. H. Collins, P. M. Lynes, and J. M. Grange, Microbiological Methods, 7th ed (Butterwort-Heinemann, Oxford, 1995), pp. 175–190.
P. A. Wayne and CLSI—Clinical and Laboratory Standards Institute, Performance Standards for Antimicrobial Disk Susceptibility Tests. Approved Standard. Document M02-A10, 10th ed (CLSI, Wayne, 2009).
K. Das, R. K. S. Tiwari, and D. K. Shrivastava (2010). J. Med. Plants Res. 4, 104–111.
A. J. R. Moller (1966). Odontolgisk Tidskrift 74, 1–38.
T. M. A. Alves, A. F. Silva, M. Brandao, T. S. M. Grandi, E. F. A. Smania Jr., A. Smania, and C. L. Zani (2010). Memorias do Instituto Oswaldo Cruz 95, 367–373.
P. A. Wayne and NCCLS, Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. Approved Standard. Document M07-A05, 5th ed (NCCLS, Wayne, 2000).
V. L. Singletone and J. A. Rossi (1996). Am. J. Enol. Vitic. 16, 144–153.
N. Venugopal and A. Mitra (2013). Appl. Surf. Sci. 85, 357–372.
G. Kaur, R. K. Verma, D. K. Rai, and S. B. Rai (2012). J. Lumin. 132, 1683–1687.
M. Darroudi, A. Khorsand Zak, M. R. Muhamad, and R. Zamiri (2014) Res. Chem. Intermed. 41, 4587–4594. doi:10.1007/s11164-014-1554-4).
K. Shameli, M. B. Ahmad, W. M. Z. W. Yunus, N. A. Ibrahim, R. A. Rahman, M. Jokar, and M. Darroudi (2010). Int. J. Nanomed. 5, 573–579.
J. A. Dahl, L. Bettye, L. S. Maddux, and J. E. Hutchison (2007). J. Chem. Rev. 107, 2228–2269.
B. Rameshbabu and G. Rajagopal (2014). IJNPR 5, (1), 34–39.
U. B. Jagtap and V. A. Bapat (2013). Ind. Crops Prod. 46, 132–137.
V. Ahluwalia, J. Kumar, R. Sisodia, and N. A. Shakil (2014). Ind. Crops Prod. 55, 202–206.
C. Jayaseelan, R. Ramkumar, A. Rahuman, and P. Perumal (2013). Ind. Crops Prod. 45, 423–429.
I. Dragieva, S. Stoeva, P. Stoimenov, E. Pavlikianov, and K. Klabunde (1999). Nanostruct. Mater. 129, 267–270.
T. Hamouda, A. Myc, B. Donovan, A. Shih, J. D. Reuter, and J. R. Baker (2001). Microbiol. Res. 156, 1–7.
P. Dibrov, J. Dzioba, K. K. Gosink, and C. C. Hase (2002). Antimicrob. Agents Chemother. 46, 2668–2670.
F. Gianluigi, F. Annarita, G. Stefania, P. Luciana, R. Mahendra, M. Giancarlo, and G. Massimiliano (2015). Molecules 20, 8856–8874.
N. A. Amro, L. P. Kotra, K. Wadu-Mesthrige, A. Bulychev, S. Mobashery, and G. Liu (2000). Langmuir 16, 2789–2796.
J. H. Crabtree, R. J. Burchette, R. A. Siddiqi, I. T. Huen, L. L. Handott, and A. Fishman (2003). Perit. Dial. Int. 23, 368–374.
S. Abuskhuna, J. Briody, M. McCann, M. Devereux, K. Kavanagh, and J. B. Fontecha (2004). Polyhedron 23, 1249–1255.
F. Furno, K. S. Morley, B. Wong, B. L. Sharp, P. L. Arnold, and S. M. Howdle (2004). J. Antimicrob. Chemother. 54, (2004), 1019–1024.
W.-Y. Huang and Y.-Z. Cai (2009). Nutr. Cancer 62, 1–20.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bankalgi, S.C., Londonkar, R.L., Madire, U. et al. Biosynthesis, Characterization and Antibacterial Effect of Phenolics-Coated Silver Nanoparticles Using Cassia javanica L.. J Clust Sci 27, 1485–1497 (2016). https://doi.org/10.1007/s10876-016-1016-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-016-1016-9