Abstract
Purpose
Human neonates are highly susceptible to a wide range of infections, which has been attributed to deficiencies in their innate and adaptive immunity. In contrast to the well-documented immaturity in neonatal adaptive immunity, deficiencies in their innate immunity are less defined. This study examined the inflammatory response of neonatal monocytes to bacterial lipopolysaccharide (LPS) and peptidoglycan (PGN) stimulation and discriminated the underlying Toll-like receptor (TLR)-mediated signal transduction pathways.
Methods
Cord blood from 30 healthy newborns of full-term elective cesarean sections and peripheral blood from 25 healthy adult volunteers were collected. Ex vivo production of inflammatory cytokines was assessed by cytometric bead array, and expression of CD14, TLR4, TLR2, phosphorylated NF-κB p65 and p38 on monocytes were detected by FACScan analysis.
Results
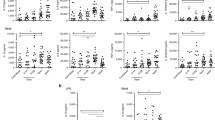
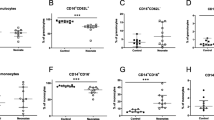
Neonatal whole blood showed significantly decreased ex vivo TNF-α and IL-1β production in response to stimulation with the TLR4 agonist LPS, but not the TLR2 agonist PGN, when compared with adult whole blood. Consistent with the diminished inflammatory cytokine response to LPS stimulation, neonatal monocytes exhibited substantially impaired TLR-mediated signal transduction pathways characterized by down-regulated expression of CD14 and TLR4, and suppressed phosphorylation of NF-κB p65 at Ser536 and p38 following LPS stimulation. In addition, neonates had a significantly lower percentage of TLR4+/CD14+ monocytes than adults.
Conclusions
These results indicate that in contrast to the adult, human neonates display deficiencies in innate immunity-associated inflammatory cytokine responses due to their defective TLR signaling pathways, which may render them more susceptible to microbial infection.



Similar content being viewed by others
Abbreviations
- BLP:
-
Bacterial lipopeptides
- ERK1/2:
-
Extracellular signal-regulated kinase 1/2
- FITC:
-
Fluorescein isothiocyanate
- IRAK:
-
IL-1 receptor-associated kinase
- JNK:
-
c-Jun NH2-terminal kinase
- LPS:
-
Lipopolysaccharide
- LTA:
-
Lipoteichoic acid
- MAPKs:
-
Mitogen-activated protein kinases
- MFI:
-
Mean fluorescence intensity
- MyD88:
-
Myeloid differentiation factor 88
- PAMPs:
-
Pathogen-associated molecular patterns
- PE:
-
Phycoerythrin
- PGN:
-
Peptidoglycan
- PMNs:
-
Polymorphonuclear neutrophils
- PRRs:
-
Pattern recognition receptors
- TLRs:
-
Toll-like receptors
- TRAF6:
-
TNF receptor-associated factor 6
References
Lawn JE, Cousens S, Zupan J. 4 million neonatal deaths: when? where? why? Lancet. 2005;365:891–900.
Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–61.
Oestergaard MZ, Inoue M, Yoshida S, et al. Neonatal mortality levels for 193 countries in 2009 with trends since 1990: a systematic analysis of progress, projections, and priorities. PLoS Med. 2011;8:e1001080.
PrabhuDas M, Adkins B, Gans H, et al. Challenges in infant immunity: implications for responses to infection and vaccines. Nat Immunol. 2011;12:189–94.
Lewis DB, Wilson CB. Developmental immunology and role of host defenses in fetal and neonatal susceptibility to infection. In: Klein JO, Remington JS, Wilson CB, Baker CJ, editors. Infections diseases of the fetus and newborn infant. 6th ed. Elsevier Saunders: Philadelphia; 2006. p. 25–128.
Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7:379–90.
Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4:553–64.
Haines CJ, Giffon TD, Lu LS, et al. Human CD4+ T cell recent thymic emigrants are identified by protein tyrosine kinase 7 and have reduced immune function. J Exp Med. 2009;206:275–85.
Opiela SJ, Koru-Sengul T, Adkins B. Murine neonatal recent thymic emigrants are phenotypically and functionally distinct from adult recent thymic emigrants. Blood. 2009;113:5635–43.
Siegrist CA, Aspinall R. B-cell responses to vaccination at the extremes of age. Nat Rev Immunol. 2009;9:185–94.
Guilmot A, Hermann E, Braud VM, Carlier Y, Truyens C. Natural killer cell responses to infections in early life. J Innate Immunol. 2011;3:280–8.
Cuenca AG, Wynn JL, Kelly-Scumpia KM, et al. Critical role for CXC ligand 10/CXC receptor 3 signaling in the murine neonatal response to sepsis. Infect Immun. 2011;79:2746–54.
Marodi L. Neonatal innate immunity to infectious agents. Infect Immun. 2006;74:1999–2006.
Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–84.
Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–8.
Takeuchi O, Hoshino K, Akira S. Cutting edge: TLR2 deficient and MyD88 deficient mice are highly susceptible to Staphlycoccus aureus infection. J Immunol. 2000;165:5392–6.
Landmann R, Muller B, Zimmerli W. CD14, new aspects of ligand and signal diversity. Microbes Infect. 2000;2:295–304.
Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–3.
Kusunoki T, Hailman E, Juan TS, Lichenstein HS, Wright SD. Molecules from Staphylococcus aureus that bind CD14 and stimulate innate immune responses. J Exp Med. 1995;182:1673–82.
Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511.
Zhang Q, Coveney AP, Yu S. Inefficient antimicrobial functions of innate phagocytes render infant mice more susceptible to bacterial infection. Eur J Immunol. 2013;43:1322–32.
Krutzik PO, Irish JM, Nolan GP, et al. Analysis of protein phosphorylation and cellular signaling events by flow cytometry: techniques and clinical applications. Clin Immunol. 2004;110:206–21.
Munoz C, Carlet J, Fitting C, et al. Dysregulation of in vitro cytokine production by monocytes during sepsis. J Clin Invest. 1991;88:1747–54.
Wynn JL, Scumpia PO, Winfield RD, et al. Defective innate immunity predisposes murine neonates to poor sepsis outcome but is reversed by TLR agonists. Blood. 2008;112:1750–8.
Kollmann TR, Crabtree J, Rein-Weston A, et al. Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol. 2009;183:7150–60.
Peters AM, Bertram P, Gahr M, Speer CP. Reduced secretion of interleukin-1 and tumor necrosis factor-alpha by neonatal monocytes. Biol Neonate. 1993;63:157–62.
Levy O. Innate immunity of the human newborn: distinct cytokine responses to LPS and other Toll-like receptor agonists. J Endotoxin Res. 2005;11:113–6.
Levy O, Zarember KA, Roy RM, et al. Selective impairment of TLR-mediated innate immunity in human newborns: neonatal blood plasma reduces monocyte TNF-alpha induction by bacterial lipopeptides, lipopolysaccharide, and imiquimod, but preserves the response to R-848. J Immunol. 2004;173:4627–34.
Caron JE, La Pine TR, Augustine NH, Martins TB, Hill HR. Multiplex analysis of toll-like receptor-stimulated neonatal cytokine response. Neonatol. 2010;97:266–73.
Yan SR, Qing G, Byers DM, et al. Role of MyD88 in diminished tumor necrosis factor alpha production by newborn mononuclear cells in response to lipopolysaccharide. Infect Immun. 2004;72:1223–9.
Tulic MK, Hodder M, Forsberg A, et al. Differences in innate immune function between allergic and nonallergic children:new insights into immune ontogeny. J Allergy Clin Immunol. 2011;127:470–8.
Liao SL, Yeh KW, Lai SH, Lee WI, Huang JL. Maturation of toll-like receptor 1–4 responsiveness during early life. Early Hum Dev. 2013;89:473–8.
Sadeghi K, Berger A, Langgartner M, et al. Immaturity of infection control in preterm and term newborns is associated with impaired toll-like receptor signaling. J Infect Dis. 2007;195:296–302.
Kopp E, Medzhitov R. Recognition of microbial infection by Toll-like receptors. Curr Opin Immunol. 2003;15:677–82.
Blander JM, Medzhitov R. Regulation of phagosome maturation by signals from Toll-like receptors. Science. 2004;304:1014–8.
Viemann D, Dubbel G, Schleifenbaum S, et al. Expression of toll-like receptors in neonatal sepsis. Pediatr Res. 2005;58:654–9.
Dasari P, Zola H, Nicholson IC. Expression of Toll-like receptors by neonatal leukocytes. Pediatr Allergy Immunol. 2011;22:221–8
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant 81272143 and 81420108022), the Natural Science Foundation of Jiangsu Province (Grant BK2011310), Jiangsu Innovation Team (Grant LJ201141), Jiangsu Province Program of Innovative and Entrepreneurial Talents (2011–2014).
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Li, Y.P., Yu, S.L., Huang, Z.J. et al. An Impaired Inflammatory Cytokine Response to Gram-Negative LPS in Human Neonates is Associated with the Defective TLR-Mediated Signaling Pathway. J Clin Immunol 35, 218–226 (2015). https://doi.org/10.1007/s10875-015-0128-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-015-0128-6