Abstract
Introduction
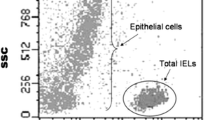
Different strategies have been developed to identify those refractory celiac disease (RCD) patients who are at risk to develop an enteropathy associated T-cell lymphoma (EATL). Flow cytometric analysis of intra-epithelial lymphocytes (IEL) with an aberrant phenotype is considered the golden standard but is not widely available. Immunohistochemistry (IHC) and T-cell receptor (TCR) rearrangement studies are commonly available but may lack sensitivity and specificity. Here, we compared the three different methods in the workup of patients suspected for RCD.
Methods
Duodenal biopsies from control patient (n = 5), RCD patients with moderately increased aberrant IEL populations (20–50 %: n = 14), and RCD patients with high numbers of aberrant IEL (>50 %: n = 5) as determined by flow cytometry were analysed by IHC and TCR-γ chain rearrangement analysis. Three pathologists scored the slides independently.
Results
Sensitivity of IHC and TCR-γ rearrangement analysis in RCD patients with high numbers of aberrant IELs was 100 and 71 %, respectively. RCD patients with aberrant cells between 25 and 50 % however, were missed by IHC and TCR in 50 and 57 % of cases, respectively. In addition, inter-rater reliability analysis of the IHC scoring revealed coder-pair Kappa coefficients between 0.28 and 0.85.
Conclusion
Immunohistochemistry and to a lesser extent TCR-γ clonality analysis are sensitive in identifying patients with high numbers of aberrant IEL populations, yet miss half of RCD patients with moderately increased numbers. In addition, IHC has a high inter-observer variability. Therefore, patients suspected for RCD should undergo flow cytometric analysis of the duodenum.


Similar content being viewed by others
References
Daum S, Cellier C, Mulder CJ. Refractory coeliac disease. Best Pract Res Clin Gastroenterol. 2005;19(3):413–24.
Nijeboer P, van Wanrooij RL, Tack GJ, Mulder CJ, Bouma G. Update on the diagnosis and management of refractory coeliac disease. Gastroenterol Res Pract. 2013;2013:518483.
Gough KR, Read AE, NAISH JM. Intestinal reticulosis as a complication of idiopathic steatorrhoea. Gut. 1962;3:232–9.
Bagdi E, Diss TC, Munson P, Isaacson PG. Mucosal intra-epithelial lymphocytes in enteropathy-associated T-cell lymphoma, ulcerative jejunitis, and refractory celiac disease constitute a neoplastic population. Blood. 1999;94(1):260–4.
Cellier C, Patey N, Mauvieux L, Jabri B, Delabesse E, Cervoni JP, et al. Abnormal intestinal intraepithelial lymphocytes in refractory sprue. Gastroenterology. 1998;114(3):471–81.
Schmitz F, Tjon JM, Lai Y, Thompson A, Kooy-Winkelaar Y, Lemmers RJ. Identification of a potential physiological precursor of aberrant cells in refractory coeliac disease type II. Gut. 2012;6:509–19.
Al-Toma A, Verbeek WH, Hadithi M, von Blomberg BM, Mulder CJ. Survival in refractory coeliac disease and enteropathy-associated T-cell lymphoma: retrospective evaluation of single-centre experience. Gut. 2007;56(10):1373–8.
Verbeek WH, Goerres MS, von Blomberg BM, Oudejans JJ, Scholten PE, Hadithi M, et al. Flow cytometric determination of aberrant intra-epithelial lymphocytes predicts T-cell lymphoma development more accurately than T-cell clonality analysis in refractory celiac disease. Clin Immunol. 2008;126(1):48–56.
Cellier C, Delabesse E, Helmer C, Patey N, Matuchansky C, Jabri B, et al. Refractory sprue, coeliac disease, and enteropathy-associated T-cell lymphoma. French Coeliac Disease Study Group Lancet. 2000;356(9225):203–8.
Daum S, Ipczynski R, Schumann M, Wahnschaffe U, Zeitz M, Ullrich R. High rates of complications and substantial mortality in both types of refractory sprue. Eur J Gastroenterol Hepatol. 2009;21(1):66–70.
Malamut G, Afchain P, Verkarre V, Lecomte T, Amiot A, Damotte D, et al. Presentation and long-term follow-up of refractory celiac disease: comparison of type I with type II. Gastroenterology. 2009;136(1):81–90.
Rubio-Tapia A, Kelly DG, Lahr BD, Dogan A, Wu TT, Murray JA. Clinical staging and survival in refractory celiac disease: a single center experience. Gastroenterology. 2009;136(1):99–107.
Di SA, Biagi F, Gobbi PG, Corazza GR. How I treat enteropathy-associated T-cell lymphoma. Blood. 2012;23:2458–68.
Tack GJ, Verbeek WH, Al-Toma A, Kuik DJ, Schreurs MW, Visser O, et al. Evaluation of Cladribine treatment in refractory celiac disease type II. World J Gastroenterol. 2011;17(4):506–13.
Rubio-Tapia A, Murray JA. Classification and management of refractory coeliac disease. Gut. 2010;59(4):547–57.
van Wanrooij RL, Schreurs MW, Bouma G, von Blomberg BM, Tack GJ, Verbeek WH. Accurate classification of RCD requires flow cytometry. Gut. 2010;30:1732.
Pallav K, Leffler DA, Tariq S, Kabbani T, Hansen J, Peer A. Noncoeliac enteropathy: the differential diagnosis of villous atrophy in contemporary clinical practice. Aliment Pharmacol Ther. 2011;6:380–90.
Tack GJ, van Wanrooij RL, Langerak AW, Tjon JM, von Blomberg BM, Heideman DA, et al. Origin and immunophenotype of aberrant IEL in RCDII patients. Mol Immunol. 2012;50(4):262–70.
de Patey-Mariaud SN, Cellier C, Jabri B, Delabesse E, Verkarre V, Roche B, et al. Distinction between coeliac disease and refractory sprue: a simple immunohistochemical method. Histopathology. 2000;37(1):70–7.
Measures of response agreement for qualitative data. Some generalizations and alternatives. Psychological Bulleting. 1976;76(5):365–77.
Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–74.
Liu H, Brais R, Lavergne-Slove A, Jeng Q, Payne K, Ye H, et al. Continual monitoring of intraepithelial lymphocyte immunophenotype and clonality is more important than snapshot analysis in the surveillance of refractory coeliac disease. Gut. 2010;59(4):452–60.
Verbeek WH, von Blomberg BM, Scholten PE, Kuik DJ, Mulder CJ, Schreurs MW. The presence of small intestinal intraepithelial gamma/delta T-lymphocytes is inversely correlated with lymphoma development in refractory celiac disease. Am J Gastroenterol. 2008;103(12):3152–8.
Malamut G, Meresse B, Cellier C, Cerf-Bensussan N. Refractory celiac disease: from bench to bedside. Semin Immunopathol 2012 Jul 19.
Lonardi S, Villanacci V, Lorenzi L, Lanzini A, Lanzarotto F, Carabellese N, et al. Anti-TCR gamma antibody in celiac disease: the value of count on formalin-fixed paraffin-embedded biopsies. Virchows Arch. 2013;463(3):409–13.
Daum S, Weiss D, Hummel M, Ullrich R, Heise W, Stein H, et al. Frequency of clonal intraepithelial T lymphocyte proliferations in enteropathy-type intestinal T cell lymphoma, coeliac disease, and refractory sprue. Gut. 2001;49(6):804–12.
Perfetti V, Brunetti L, Biagi F, Ciccocioppo R, Bianchi PI, Corazza GR. TCRbeta Clonality Improves Diagnostic Yield of TCRgamma Clonality in Refractory Celiac Disease. J Clin Gastroenterol 2012 Jan 30.
Authorship Contributions
RLJVW designed and conducted the study, performed laboratory studies and wrote the manuscript. DMJM performed laboratory studies.
EANB, JM and LGK evaluated histological slides.
HJB and BMEVB performed and evaluated flow cytometric data.
DAMH performed and evaluated clonality analysis.
GB and CJJM designed and supervised the study, and wrote the manuscript.
Funding
This work was supported by the Coeliac Disease Consortium, The Netherlands.
Competing Interest
None of the authors states a conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van Wanrooij, R.L.J., Müller, D.M.J., Neefjes-Borst, E.A. et al. Optimal Strategies to Identify Aberrant Intra-Epithelial Lymphocytes in Refractory Coeliac Disease. J Clin Immunol 34, 828–835 (2014). https://doi.org/10.1007/s10875-014-0075-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-014-0075-7