Abstract
Introduction
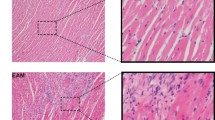
Experimental autoimmune myocarditis (EAM) is mediated by myocardial infiltration by myosin-specific T cells secreting inflammatory cytokines.
Materials and methods
To clarify the role of cytokines in EAM, we compared STAT 6-deficient (−/−) with STAT 4−/− and wild-type (BALB/CJ) mice following immunization with cardiac myosin peptide (614–629).
Results
Wild-type mice developed severe disease with a small increase in severity in STAT 6−/− mice, while STAT 4−/− mice were resistant to EAM. STAT 6−/− mice had increased splenocyte proliferation and INF-γ production versus wild type, while STAT 4−/− mice had decreased proliferation and INF-γ. Following oral administration of myosin (614–629), tolerization was induced in wild-type mice evidenced by amelioration of myocarditis and up-regulation of IL-4. Adoptive transfer of splenocytes from orally tolerized mice resulted in inhibition of disease in STAT 6−/− mice.
Conclusion
These results demonstrate that oral tolerization ameliorates EAM in BALB/CJ mice and indicate a down-regulatory role for STAT 6 genes.




Similar content being viewed by others
References
Caforio AL, Mahon NJ, Tona F, McKenna WT. Circulating cardiac autoantibodies in dilated cardiomyopathy and myocarditis: pathogenetic and clinical significance. Eur J Heart Fail. 2002;4(4):411–7. doi:10.1016/S1388-9842(02)00010-7.
Figulda HR. Transformation of myocarditis and inflammatory cardiomyopathy to idiopathic dilated cardiomyopathy to idiopathic dilated cardiomyopathy: facts and fiction. Med Microbiol Immunol (Berl). 2004;193:61–4. doi:10.1007/s00430-003-0205-y.
Frustaci A, Chimenti C, Calabrese F, Pieroni M, Thiene G, Maseri A. Immunosuppressive therapy for active lymphocytic myocarditis: virological and immunological profile of responders versus nonresponders. Circulation. 2003;107(6):857–63. doi:10.1161/01.CIR.0000048147.15962.31.
Smith SC, Allen PM. Myosin-induced acute myocarditis is a T cell-mediated disease. J Immunol. 1991;147(2):2141–7.
Kishimoto C, Hiraoka Y, Takamatsu N, Takada H, Kamiya H, Ochiai H. An in vivo model of autoimmune post coxsackievirus B3 myocarditis in severe combined immunodeficiency mouse. Cardiovasc Res. 2003;60(2):397–403. doi:10.1016/j.cardiores.2003.07.002.
Penninger JM, Pummerer C, Lui P, Neu N, Bachmaier K. Cellular and molecular mechanisms of murine myocarditis. APMIS. 1997;105:1–13.
Huber SA. Animal models of human disease. Autoimmunity in myocarditis: relevance of animal models. Clin Immunol Immunopathol. 1997;83(2):93–102. doi:10.1006/clin.1997.4342.
Neu N, Rose NR, Beisel KW, Herskovitz A, Gurri-Glass G, Craig SW. Cardiac myosin induces myocarditis in genetically predisposed mice. J Immunol. 1987;139:3630–6.
Pummerer CL, Luze K, Grassl G, Bachmaier K, Offner F, Burrell SK, et al. Identification of cardiac myosin peptides capable of inducing autoimmune myocarditis in BALB\CJ mice. J Clin Invest. 1996;97(9):2057–62. doi:10.1172/JCI118642.
Bachmaier K, Neu N, dela Maza LM, Pal S, Hessel A, Penninger JM. Chlamydia infections and heart disease linked through antigenic mimicry. Science. 1999;283:1335–13339. doi:10.1126/science.283.5406.1335.
Cunningham MW. T regulatory cells: Sentinels against autoimmune heart disease. Circ Res. 2006;99:1024–6. doi:10.1161/01.RES.0000250832.30969.6a.
Faria AMC, Weiner HL. Oral tolerance mechanisms and therapeutic applications. Adv Immunol. 1999;73:153–264. doi:10.1016/S0065-2776(08)60787-7.
Bryant D, Becker L, Richardson J, Shelton J, Franco F, Peshock R, et al. Cardiac failure in transgenic mice with myocardial expression of tumor necrosis factor-alpha. Circulation. 1998;97:1375–81.
Afanasyeva M, Wang Y, Kaya Z, Stafford EA, Dohmen KM, Sadighi Akha AA, et al. Interleukin-12 receptor/STAT 4 signaling is required for the development of autoimmune myocarditis in mice by an interferon-gamma-independent pathway. Circulation. 2001;104:3145–51. doi:10.1161/hc5001.100629.
Metcalf D, DiRago L, Mifsud A, Hartley L, Alexander WS. The development of fatal myocarditis and polymyositis in mice heterozygous for INF-γ and lacking the SOCS-1 gene. Proc Natl Acad Sci U S A. 2000;79:9174–9. doi:10.1073/pnas.160255197.
Smith SC, Allen PM. Neutralization of endogenous tumor necrosis factor ameliorates the severity of myosin-induced myocarditis. Circ Res. 1992;70(4):856–63.
Bachmaier K, Pummerer C, Kozieradzki I, Pfeffer K, Mak TW, Neu N, et al. Low molecular weight tumor necrosis factor receptor p55 controls induction of autoimmune heart disease. Circulation. 1997;95(3):655–61.
Eriksson U, Kurrer MO, Bengasser R, Eugster HP, Saremaslami P, Follath F, et al. Lethal autoimmune myocarditis in INF-γ receptor deficient mice: enhanced disease severity impaired by iNOS induction. Circulation. 2001;103(1):18–21.
Eriksson U, Kurrer MO, Sebald W, Brombacher F, Kopf M. Dual role of the IL-12/INF-γ axis in the development of myocarditis: induction of IL-12 and protection by INF-γ. J Immunol. 2001;167:5464–9.
Feuerer M, Eulenburg K, Loddenkemper C, Hamann A, Huehn J. Self-limitation of Th1-mediated inflammation by INF-γ. J Immunol. 2006;176:2857–63.
Valaperti A, Marty RR, Kania G, Germano D, Mauermann N, Dirnhofer S, et al. CD 11b+ monocytes abrogate Th17 CD4 + T cell-mediated experimental autoimmune myocarditis. J Immunol. 2008;180:2686–95.
Rangachari M, Mauermann M, Marty RR, Dirnhofer S, Kurrer MO, Komnenovic V, et al. T-bet negatively regulates autoimmune myocarditis by suppressing local production of interleukin 17. J Exp Med. 2006;203:2009–19. doi:10.1084/jem.20052222.
Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6(11):1069–70. doi:10.1038/ni1261.
Sonderegger I, Rohn TA, Kurrer MO, Iezzi G, Zou Y, Kastelein RA, et al. Neutralization of IL-17 by active vaccination inhibits IL-23-dependent autoimmune myocarditis. Eur J Immunol. 2006;36:2849–56. doi:10.1002/eji.200636484.
Afanasyeva M, Wang Y, Kaya Z, Park S, Zilliox MJ, Schofield BH, et al. Experimental autoimmune myocarditis in A/J mice is an interleukin-4 dependent disease with a Th2 phenotype. Am J Pathol. 2001;159:193–203.
Cihakova D, Barin JG, Afanasyeva M, Kimura M, Fairweather D, Berg M, et al. Interleukin-13 protects against experimental autoimmune myocarditis by regulating macrophage differentiation. Am J Pathol. 2008;172:1195–208. doi:10.2353/ajpath.2008.070207.
Imada K, Leonard WJ. The JAK-STAT pathway. Mol Immunol. 2000;37(1-2):1–11. doi:10.1016/S0161-5890(00)00018-3.
Thierfelder WE, van Deursen JM, Yamamoto K, Tripp RA, Sarawar SR, Carson RT, et al. Requirement for STAT 4 in interleukin-12-mediated responses of natural killer and T cells. Nature. 1996;382:171–4. doi:10.1038/382171a0.
Kaplan MH, Sun YL, Hoey T, Grusby MJ. Impaired IL-12 responses and enhanced development of Th2 cells in STAT 4-deficient mice. Nature. 1996;382:171–4. doi:10.1038/382174a0.
Takeda K, Tanaka T, Shi W, Matsumoto M, Minami M, Kashiwamura S, et al. Essential role of STAT 6 in IL-4 signaling. Nature. 1996;380:627–30. doi:10.1038/380627a0.
Kaplan MH, Schinder U, Smiley ST, Grusby MJ. STAT 6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313–9. doi:10.1016/S1074-7613(00)80439-2.
Holz A, Bot A, Coon B, Wolfe T, Grusby MJ, von Herrath MG. Disruption of STAT 4 signaling pathway protects from autoimmune diabetes while retaining antiviral immune competence. J Immunol. 1999;163:5374–82.
Chitnis T, Najafian N, Benou C, Salama AD, Grusby M, Sayegh MH, et al. Effect of targeted disruption of STAT 4 and STAT 6 on the induction of experimental autoimmune encephalomyelitis. J Clin Invest. 2001;108:739–47.
Frisancho-Kiss S, Nyland JF, Davis SE, Frisancho JA, Barrett MA, Rose NR, et al. Sex differences in coxsackievirus B3-induced myocarditis: IL-12Rbeta 1 signaling and INF-γ increase inflammation in males independent from STAT4. Brain Res. 2006;26(1):139–47. doi:10.1016/j.brainres.2006.08.003.
Kaplan MH, Wurster AL, Grusby MJ. A signal transducer and activator of transcription (STAT) 4-independent pathway for the development of T helper type 1 cells. J Exp Med. 1998;188:1191–6. doi:10.1084/jem.188.6.1191.
Kaya Z, Dohmen KM, Wang Y, Afanasyeva M, Leuschner F, Rose NR. Cutting edge: a critical role for IL-10 in induction of nasal tolerance in experimental autoimmune myocarditis. J Immunol. 2002;168(4):1552–6.
Chen Y, Inobe J, Weiner HL. Induction of oral tolerance to myelin basic protein in CD8-depleted mice: both CD4+ and CD8+ cells mediate active suppression. J Immunol. 1995;155:910–6.
Gonnella PA, Chen Y, Inobe J, Komagata Y, Quartulli M, Weiner HL. In situ immune response in gut-associated lymphoid tissue (GALT) following oral antigen in TCR-transgenic mice. J Immunol. 1998;160(10):4708–18.
Gonnella PA, Waldner HP, Weiner HL. B cell-deficient mice (µMT) have alterations in the cytokine microenvironment of the gut associated lymphoid tissue (GALT) and a defect in the low dose mechanism of oral tolerance. J Immunol. 2001;166(7):4456–64.
Homann D, Holz A, Bot A, Coon B, Wolfe T, Peterson J, et al. Autoreactive CD4 + T cells protect from autoimmune diabetes via bystander suppression using the IL-4/Stat6 pathway. Immunity. 1999;11:463–72. doi:10.1016/S1074-7613(00)80121-1.
Fairweather D, Frisancho S, Yusung SA, Barrett MA, Davis SE, Steele RA, et al. IL-12 protects against coxsackie B3-induced myocarditis by increasing INF-γ and macrophage and neutrophil populations in the heart. J Immunol. 2005;174:261–9.
Jin D, Takamoto M, Hu T, Taki S, Sugane K. STAT6 signalling is important in CD8 (+) T-cell activation and defence against Toxoplasma gondii infection in the brain. Immunology. 2009; doi:10.1111/j.1365-2567.2008.02935.x.
Kohyama M, Sugahara D, Hosokawa H, Kubo M, Hozumi N. IL-4 mediated development of TGF-beta 1-producing cells from naïve CD4+ T cells through a STAT 6-independent mechanism. Eur J Immunol. 2001;12:3659–66. doi:10.1002/1521-4141(200112) 31:12<3659::AID-IMMU3659>3.0.CO;2-6.
Lee YH, Shin DW, Kasper LH. Sequential analysis of cell differentials and INF-γ production of splenocytes from mice infected with Toxoplasma gondii. Korean J Parasitol. 2000;38(2):85–90.
Gonnella PA, Waldner HP, Del Nido PJ, McGowan FX. Inhibition of experimental autoimmune myocarditis: Peripheral deletion of TcR Vβ 8.1, 8.2+CD4+ T cells in TLR-4 deficient mice. J Autoimmun. 2008;31(2):180–7. doi:10.1016/j.jaut.2008.06.002.
Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;241:744–8. doi:10.1038/nature01355.
Cihakova D, Rose NR. Pathogenesis of myocarditis and dilated cardiomyopathy. Adv Immunol. 2008;99:95–114. doi:10.1016/S0065-2776(08)00604-4.
Wiekowski MT, Leach MW, Evans EW, Sullivan L, Chen SC, Vassileva G, et al. Ubiquitious transgenic expression of the IL-23 subunit p19 induces multiorgan inflammation, runting infertility and premature death. J Immunol. 2001;166:7563–70.
Ha S, Kim D, Baek K, Yun Y, Sung Y. IL-23 induces stronger sustained CTL and Th1 immune responses than IL-12 in hepatitis C virus envelope protein 2 DNA immunization. J Immunol. 2004;172:525–31.
Powell MB, Michell D, Lederman J, Buckmeier J, Zamvil SS, Graham M, et al. Lymphotoxin and tumor necrosis factor-alpha production by myelin basic protein-specific T cell clones correlates with encephalitogenicity. Int Immunol. 1990;2:539–44. doi:10.1093/intimm/2.6.539.
Begolka WS, Vanderlugt CL, Rahbe SM, Miller SD. Differential expression of inflammatory cytokines parallels progression of central nervous system pathology in two clinically distinct models of multiple sclerosis. J Immunol. 1998;161:4437–46.
Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177(1):566–73.
Issazadeh S, Navikas V, Schaub M, Sayegh M, Khoury S. Kinetics of expression of costimulatory molecules and their ligands in murine relapsing experimental autoimmune encephalomyelitis in vivo. J Immunol. 1998;161:1104–12.
Khoury SJ, Hancock WW, Weiner HL. Oral tolerance to myelin basic protein and natural recovery from experimental autoimmune encephalomyelitis associated with downregulation of inflammatory cytokines and differential upregulation of transforming growth factor beta, interleukin 4 and prostaglandin E expression in the brain. J Exp Med. 1992;176:1355–64. doi:10.1084/jem.176.5.1355.
Fairweather D, Yusung S, Frisancho S, Barrett M, Gatewood S, Steele R, et al. IL-12 receptor β1 and toll-like receptor 4 increase IL-1β and IL-18-associated myocarditis and coxsackie virus replication. J Immunol. 2003;170:4731–7.
Acknowledgments
This work was supported by grant # HL052589 from the NIH.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gonnella, P.A., Del Nido, P.J. & McGowan, F.X. Oral Tolerization with Cardiac Myosin Peptide (614–629) Ameliorates Experimental Autoimmune Myocarditis: Role of Stat 6 Genes in BALB/CJ Mice. J Clin Immunol 29, 434–443 (2009). https://doi.org/10.1007/s10875-009-9290-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-009-9290-z