Abstract
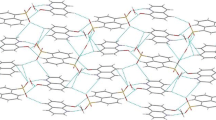
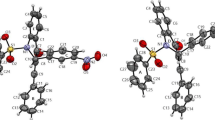
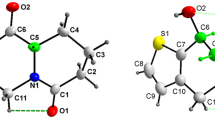
The crystalline compounds quinolin-8-ol (1) and its two salts 2, and 3 have been prepared and structurally characterized. Compound 1 crystallizes in the triclinic, space group P-1, with a = 6.5775(5) Å, b = 7.8285(6) Å, c = 14.4617(13) Å, α = 96.099(2), β = 92.4700(10)°, γ = 106.171(3), V = 709.09(10) Å3, Z = 4. Compound 1 is a new polymorph of quinolin-8-ol, and displays as dimer. Compound 2 crystallizes in the monoclinic, space group P2(1)/n, with a = 7.4815(11) Å, b = 18.844(3) Å, c = 8.0396(8) Å, β = 94.069(10), V = 1130.5(3) Å3, Z = 4. Compound 3 crystallizes in the triclinic, space group P-1, with a = 7.0968(6) Å, b = 8.6854(7) Å, c = 11.0494(11) Å, α = 105.169(2), β = 90.3700(10)°, γ = 106.031(2), V = 629.50(10) Å3, Z = 1. In the compound 3, the middle two COOH groups were ionized. In the compounds 2 and 3, as predicted on the basis of pKa differences, proton transfer from the COOH group to the hetero-nitrogen of the quinolin-8-ol molecule occurs, resulting in the formation of the hydrogen or dihydrogen salts. In neither of the compounds (2 and 3) the primary cyclic hydrogen-bonded R 22 (8) A–B heterodimer was formed, involving the second oxygen of the anion and the 8-hydroxy substituent of quinolin-8-ol. Instead, this molecule acts in a bridging mode to link the associated molecular units into chain polymers via combination of hydrogen bonds and other nonbonding interactions. The role of these non-covalent interactions in the crystal packing is analyzed. Under these weak interactions, compounds 2 and 3 displayed 3D framework structures.
Graphical Abstract
The crystal structures of quinolin-8-ol and its two salts from acetylenedicarboxylic acid, and butane-1,2,3,4-tetracarboxylic acid display extensive classical hydrogen bonding as well as other non-covalent CH–O, CH2–O, O–Cπ, CH–Cπ, and π···π interactions, giving 0/3D framework structures.









Similar content being viewed by others
References
Tiekink ERT, Vittal JJ, Zaworotko MJ (2010) In organic crystal engineering: frontiers in crystal engineering. Wiley, Chichester
Tanase S, Bouwman E, Long GJ, Shahin AM, Mills AM, Jan Reedijk ALS (2004) Eur J Inorg Chem 23:4572
Janiak C (2000) J Chem Soc Dalton Trans 21:3885
Takahashi O, Kohno Y, Nishio M (2010) Chem Rev 110:6049
Berkovitch-Yellin Z, Leiserowitz L (1984) Acta Cryst B40:159
Cho KH, No KT, Scheraga HA (2000) J Phys Chem A 104:6505
Koch W, Frenking G, Gauss J, Cremer D (1986) J Am Chem Soc 108:5808
Etter MC (1990) Acc Chem Res 23:120
Braga D, Grepioni F, Desiraju GR (1998) Chem Rev 98:1375
Desiraju GR (2002) Acc Chem Res 35:565
Zaworotko MJ (2007) Cryst Growth Des 7:4
Maamen M, Gordon DM (1995) Acc Chem Res 28:37 (and references therein)
Weyna DR, Shattock T, Vishweshwar P, Zaworotko MJ (2009) Cryst Growth Des 9:1106
Du M, Zhang ZH, Zhao XJ (2005) Cryst Growth Des 5:1247
Desiraju GR (1989) Crystal engineering, the design of organic solids. Elsevier, Amsterdam
Leiserowitz L (1976) Acta Crystallogr B 32:775
Kolotuchin SV, Fenlon EE, Wilson SR, Loweth CJ, Zimmerman SC (1995) Angew Chem Int Ed Engl 34:2654
Kuduva SS, Craig DC, Nangia A, Desiraju GR (1999) J Am Chem Soc 121:1936
Bernstein J, Etter MC, Leiserowitz L (1994) Struct Correl 2:431
Moulton B, Zaworotko MJ (2001) Chem Rev 101:1629
Reddy LS, Bethune SJ, Kampf JW, Rodríguez-Hornedo N (2009) Cryst Growth Des 9:378
Lee IS, Shin DM, Chung YK (2003) Cryst Growth Des 3:521
Bhogala BR, Nangia A (2003) Cryst Growth Des 3:547
MacDonald JC, Dorrestein PC, Pilley MM (2001) Cryst Growth Des 1:29
Highfill ML, Chandrasekaran A, Lynch DE, Hamilton DG (2002) Cryst Growth Des 2:15
Vishweshwar P, Nangia A, Lynch VM (2002) J Org Chem 67:556
Nichol GS, Clegg W (2009) Cryst Growth Des 9:1844
Men YB, Sun JL, Huang ZT, Zheng QY (2009) CrystEngComm 11:978
Smith G, Wermuth UD, White JM (2001) Aust J Chem 54:171
Smith G, Wermuth UD, White JM (2003) CrystEngComm 5:58
Smith G, White JM (2001) Aust J Chem 54:97
Jin SW, Zhang WB, Wang DQ, Gao HF, Zhou JZ, Chen RP, Xu XL (2010) J Chem Crystallogr 40:87
Jin SW, Wang DQ, Jin ZJ, Wang LQ (2009) Pol J Chem 83:1937
Bruker (2004) SMART and SAINT. Bruker AXS, Madison
Sheldrick GM (2000) SHELXTL, structure determination software suite, version 6.14. Bruker AXS, Madison
Wendlandt WW, Horton GR (1963) J Inorg Nucl Chem 25:247
Roychowdhury P, Das BN, Basak BS (1978) Acta Crystallogr Sect B 34:1047
Simonsen SH, Bechtel DW (1980) Am Cryst Assoc Ser 27:23
Bannerjee T, Saha NN (1986) Acta Crystallogr Sect C 42:1408
Timofeeva TV, Kuhn GH, Nesterov VV, Nesterov VN, Frazier DO, Penn BG, Antipin MY (2003) Cryst Growth Des 3:383
Banerjee T, Saha NN (1986) Acta Cryst C42:1408
Castañeda R, Antal SA, Draguta S, Timofeeva TV, Khrustalev VN (2014) Acta Cryst E70:o924
Smith G, Wermuth UD, Healy PC, White JM (2006) Acta Cryst E62:o5089
Eichstaedt K, Olszewska T, Gdaniec M (2013) Acta Cryst E69:o144
Jin SW, Wang DQ, Liang SS, Chen SJ (2012) J Chem Crystallogr 42:759
Najafpour MM, Holynska M, Lis T (2008) Acta Cryst E64:o985
Barnes HA, Barnes JC (1996) Acta Cryst C52:731
McKee V, Najafpour MM (2007) Acta Cryst E63:o741
Etter MC, MacDonald JC, Bernstein J (1990) Acta Cryst B46:256
Acknowledgments
This research was supported by Zhejiang Provincial Natural Science Foundation of China under Grant No. LY14B010006, the Education Office Foundation of Zhejiang Province under Grant No. Y201017321, the National Training Programs of Innovation and Entrepreneurship of China for Undergraduates under Grant No. 201410341022, and the Zhejiang A & F University Science Foundation under Grant No. 2009FK63.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, K., Jin, S., Wang, D. et al. Crystal and Molecular Structures of Quinolin-8-ol and its Salts with Acetylenedicarboxylic Acid, and Butane-1,2,3,4-tetracarboxylic Acid. J Chem Crystallogr 45, 290–299 (2015). https://doi.org/10.1007/s10870-015-0590-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-015-0590-2