Abstract
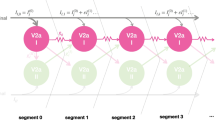
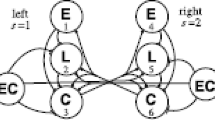
Voluntary movements in animals are often episodic, with abrupt onset and termination. Elevated neuronal excitation is required to drive the neuronal circuits underlying such movements; however, the mechanisms that sustain this increased excitation are largely unknown. In the medicinal leech, an identified cascade of excitation has been traced from mechanosensory neurons to the swim oscillator circuit. Although this cascade explains the initiation of excitatory drive (and hence swim initiation), it cannot account for the prolonged excitation (10–100 s) that underlies swim episodes. We present results of physiological and theoretical investigations into the mechanisms that maintain swimming activity in the leech. Although intrasegmental mechanisms can prolong stimulus-evoked excitation for more than one second, maintained excitation and sustained swimming activity requires chains of several ganglia. Experimental and modeling studies suggest that mutually excitatory intersegmental interactions can drive bouts of swimming activity in leeches. Our model neuronal circuits, which incorporated mutually excitatory neurons whose activity was limited by impulse adaptation, also replicated the following major experimental findings: (1) swimming can be initiated and terminated by a single neuron, (2) swim duration decreases with experimental reduction in nerve cord length, and (3) swim duration decreases as the interval between swim episodes is reduced.














Similar content being viewed by others
Abbreviations
- ADO:
-
Adenosine
- BPE:
-
Bursts per episode
- DP:
-
Dorsal posterior (nerve)
- CNS:
-
Central nervous system
- CPG:
-
Central pattern generator
- IN:
-
Interneuron
- MN:
-
Motoneurons
- RCI:
-
Recurrent cyclic inhibition
- RE:
-
Reciprocally excitatory
References
Ikeda, K., Wiersma, C.A.: Autogenic rhythmicity in the abdominal ganglia of the crayfish: the control of swimmeret movements. Comp. Biochem. Physiol. 12, 107–115 (1964)
Mulloney, B.: During fictive locomotion, graded synaptic currents drive bursts of impulses in swimmeret motor neurons. J. Neurosci. 23, 5953–5962 (2003)
Wilson, D.M.: The central nervous control of flight in a locust. J. Exp. Biol. 38, 471–490 (1961)
Thompson, S., Watson, W.H., III: Central pattern generator for swimming in Melibe. J. Exp. Biol. 208, 1347–1361 (2005)
Katz, P.S., Frost, W.N.: Intrinsic neuromodulation in the Tritonia swim CPG: the serotonergic dorsal swim interneurons act presynaptically to enhance transmitter release from interneuron C2. J. Neurosci. 15, 6035–6045 (1995)
Katz, P.S.: Intrinsic and extrinsic neuromodulation of motor circuits. Curr. Opin. Neurobiol. 5, 799–808 (1995)
Satterlie, R.A.: Reciprocal inhibition and rhythmicity: swimming in a pteropod mollusk. In: Jacklet, J.W. (ed.) Cellular and Neuronal Oscillators, pp. 151–171 (1989)
Jing, J., Gillette, R.: Escape swim network interneurons have diverse roles in behavioral switching and putative arousal in Pleurobranchaea. J. Neurophysiol. 83, 1346–1355 (2000)
Hagevik, A., McClellan, A.D.: Coordination of locomotor activity in the lamprey: role of descending drive to oscillators along the spinal cord. Exp. Brain Res. 128, 481–490 (1999)
Masino, M.A., Fetcho, J.R.: Fictive swimming motor patterns in wild type and mutant larval zebrafish. J. Neurophysiol. 93, 3177–3188 (2005)
Grillner, S., Wallen, P.: Cellular bases of a vertebrate locomotor system-steering, intersegmental and segmental co-ordination and sensory control. Brain Res. Rev. 40, 92–106 (2002)
Frost, W.N., Katz, P.S.: Single neuron control over a complex motor program. Proc. Natl. Acad. Sci. USA 93, 422–426 (1996)
Magnuson, D.S., Trinder, T.C.: Locomotor rhythm evoked by ventrolateral funiculus stimulation in the neonatal rat spinal cord in vitro. J. Neurophysiol. 77, 200–206 (1997)
Kristan, W.B., Jr., Calabrese, R.L., Friesen, W.O.: Neuronal control of leech behavior. Prog. Neurobiol. 76, 279–327 (2005)
Marder, E., Calabrese, R.L.: Principles of rhythmic motor pattern generation. Physiol. Rev. 76, 687–717 (1996)
Major, G., Tank, D.: Persistent neural activity: prevalence and mechanisms. Curr. Opin. Neurobiol. 14, 675–684 (2004)
Friesen, W.O., Mullins, O.J., Hackett, J.T.: Neuronal models for the initiation and termination of animal locomotion. Neuroscience Meeting Planner Program No. 859.6, Society for Neuroscience, Chicago, Il. (2009)
Brown, P., Dale, N.: Spike-independent release of ATP from Xenopus spinal neurons evoked by activation of glutamate receptors. J. Physiol. 540, 851–860 (2002)
Brown, P., Dale, N.: Modulation of K(+) currents in Xenopus spinal neurons by p2y receptors: a role for ATP and ADP in motor pattern generation. J. Physiol. 540, 843–850 (2002)
Brown, P., Dale, N.: Adenosine A1 receptors modulate high voltage-activated Ca2 + currents and motor pattern generation in the Xenopus embryo. J. Physiol. 525, 655–667 (2000)
Dale, N.: Resetting intrinsic purinergic modulation of neural activity: an associative mechanism? J. Neurosci. 22, 10461–10469 (2002)
Dale, N.: Delayed production of adenosine underlies temporal modulation of swimming in frog embryo. J. Physiol. 511, 265–272 (1998)
Dale, N., Gilday, D.: Regulation of rhythmic movements by purinergic neurotransmitters in frog embryos. Nature 383, 259–263 (1996)
Dale, N., Kuenzi, F.M.: Ion channels and the control of swimming in the Xenopus embryo. Prog. Neurobiol. 53, 729–756 (1997)
Viana Di Prisco, G., Pearlstein, E., Robitaille, R., Dubuc, R.: Role of sensory-evoked NMDA plateau potentials in the initiation of locomotion. Science 278, 1122–1125 (1997)
Viana Di Prisco, G., Pearlstein, E., Le Ray, D., Robitaille, R., Dubuc, R.: A cellular mechanism for the transformation of a sensory input into a motor command. J. Neurosci. 20, 8169–8176 (2000)
Li, W.C., Soffe, S.R., Wolf, E., Roberts, A.: Persistent responses to brief stimuli: feedback excitation among brainstem neurons. J. Neurosci. 26, 4026–4035 (2006)
Li, W.C., Roberts, A., Soffe, S.R.: Locomotor rhythm maintenance: electrical coupling among premotor excitatory interneurons in the brainstem and spinal cord of young Xenopus tadpoles. J. Physiol. 587, 1677–1693 (2009)
Brodfuehrer, P.D., Friesen, W.O.: From stimulation to undulation: a neuronal pathway for the control of swimming in the leech. Science 234, 1002–1004 (1986)
Brodfuehrer, P.D., Friesen, W.O.: Initiation of swimming activity by trigger neurons in the leech subesophageal ganglion. I. Output connections of Tr1 and Tr2. J. Comp. Physiol. A. 159, 489–502 (1986)
Brodfuehrer, P.D., Friesen, W.O.: Initiation of swimming activity by trigger neurons in the leech subesophageal ganglion. III. Sensory inputs to Tr1 and Tr2. J. Comp. Physiol. A. 159, 511–519 (1986)
Stent, G.S., Kristan, W.B., Jr, Friesen, W.O., Ort, C.A., Poon, M., Calabrese, R.L.: Neuronal generation of the leech swimming movement. Science 200, 1348–1357 (1978)
Taylor, A.L., Cottrell, G.W., Kleinfeld, D., B., K.W., Jr: Imaging reveals synaptic targets of a swim-terminating neuron in the leech CNS. J. Neurosci. 23, 11402–11410 (2003)
Brodfuehrer, P.D., Parker, H.J., Burns, A., Berg, M.: Regulation of the segmental swim-generating system by a pair of identified interneurons in the leech head ganglion. J. Neurophysiol. 73, 983–992 (1995)
Esch, T., Mesce, K.A., Kristan, W.B.: Evidence for sequential decision making in the medicinal leech. J. Neurosci. 22, 11045–11054 (2002)
Weeks, J.C., Jr., Kristan, W.B.: Initiation, maintenance and modulation of swimming in the medicinal leech by the activity of a single neurone. J. Exp. Biol. 77, 71–88 (1978)
Weeks, J.C.: Segmental specialization of a leech swim-initiating interneuron, cell 205. J. Neurosci. 2, 972–985 (1982)
Debski, E.A., Friesen, W.O.: Intracellular stimulation of sensory cells elicits swimming activity in the medicinal leech. J. Comp. Physiol., A. 160, 447–457 (1987)
Nusbaum, M.P., Friesen, W.O., Kristan, W.B., Jr, Pearce, R.A.: Neural mechanisms generating the leech swimming rhythm: swim-initiator neurons excite the network of swim oscillator neurons. J. Comp. Physiol., A. 161, 355–366 (1987)
Friesen, W.O., Hocker, C.G.: Functional analyses of the leech swim oscillator. J. Neurophysiol. 86, 824–835 (2001)
Friesen, W.O.: Neuronal control of leech swimming movements. I. Inhibitory interactions between motor neurons. J. Comp. Physiol., A. 166, 195–203 (1989)
Kristan, W.B., Jr., Calabrese, R.L.: Rhythmic swimming activity in neurones of the isolated nerve cord of the leech. J. Exp. Biol. 65, 643–668 (1976)
Brodfuehrer, P.D., Kogelnik, A.M., Friesen, W.O., Cohen, A.H.: Effect of the tail ganglion on swimming activity in the leech. Behav. Neural Biol. 59, 162–166 (1993)
Hocker, C.G., Yu, X., Friesen, W.O.: Functionally heterogeneous segmental oscillators generate swimming in the medical leech. J. Comp. Physiol. A 186, 871–883 (2000)
Friesen, W.O., Stent, G.S.: Generation of a locomotory rhythm by a neural network with recurrent cyclic inhibition. Biol. Cybern. 28, 27–40 (1977)
Pearce, R.A., Friesen, W.O.: A model for intersegmental coordination in the leech nerve cord. Biol. Cybern. 58, 301–311 (1988)
Cang, J., Friesen, W.O.: Model for intersegmental coordination of leech swimming: central and sensory mechanisms. J. Neurophysiol. 87, 2760–2769 (2002)
Zheng, M., Friesen, W.O., Iwasaki, T.: Systems-level modeling of neuronal circuits for leech swimming. J. Comput. Neurosci. 22, 21–38 (2007)
Friesen, W.O., Friesen, J.A.: Neurodynamix II: Concepts of Neurophysiology Illustrated by Computer Simulations, pp. 228. Oxford University Press, New York (2010)
Friesen, W.O., Poon, M., Stent, G.S.: Neuronal control of swimming in the medicinal leech. IV. Identification of a network of oscillatory interneurones. J. Exp. Biol. 75, 25–43 (1978)
Poon, M., Friesen, W.O., Stent, G.S.: Neuronal control of swimming in the medicinal leech. V. Connections between the oscillatory interneurones and the motor neurones. J. Exp. Biol. 75, 45–63 (1978)
O’Gara, B.A., Friesen, W.O.: Termination of leech swimming activity by a previously identified swim trigger neuron. J. Comp. Physiol. A. 177, 627–636 (1995)
Granzow, B., Friesen, W.O., Kristan, W.B., Jr.: Physiological and morphological analysis of synaptic transmission between leech motor neurons. J. Neurosci. 5, 2035–2050 (1985)
Yu, X., Nguyen, B., Friesen, W.O.: Sensory feedback can coordinate the swimming activity of the leech. J. Neurosci. 19, 4634–4643 (1999)
Angstadt, J.D., Friesen, W.O.: Modulation of swimming behavior in the medicinal leech. I. Effects of serotonin on the electrical properties of swim-gating cell 204. J. Comp. Physiol., A. 172, 223–234 (1993)
Weeks, J.C.: Neuronal basis of leech swimming: separation of swim initiation, pattern generation, and intersegmental coordination by selective lesions. J. Neurophysiol. 45, 698–723 (1981)
Weeks, J.C.: Synaptic basis of swim initiation in the leech. I. Connections of a swim-initiating neuron (cell 204) with motor neurons and pattern-generating ‘oscillator’ neurons. J. Comp. Physiol., A. 148, 253–263 (1982)
Hashemzadeh-Gargari, H., Friesen, W.O.: Modulation of swimming activity in the medicinal leech by serotonin and octopamine. Comp. Biochem. Physiol. 94, 295–302 (1989)
Tian, J., Iwasaki, T., Friesen, W.O.: Analysis of impulse adaptation in motoneurons. J. Comp. Physiol., A 196, 123–136 (2010)
Kristan, W.B., Jr., McGirr, S.J., Simpson, G.V.: Behavioural and mechanosensory neurone responses to skin stimulation in leeches. J. Exp. Biol. 96, 143–160 (1982)
Roberts, A., Li, W.C., Soffe, S.R., Wolf, E.: Origin of excitatory drive to a spinal locomotor network. Brain Res. Rev. 57, 22–28 (2008)
Parker, D., Grillner, S.: The activity-dependent plasticity of segmental and intersegmental synaptic connections in the lamprey spinal cord. Eur. J. Neurosci. 12, 2135–2146 (2000)
Cangiano, L., Grillner, S.: Fast and slow locomotor burst generation in the hemispinal cord of the lamprey. J. Neurophysiol. 89, 2931–2942 (2003)
Cangiano, L., Grillner, S.: Mechanisms of rhythm generation in a spinal locomotor network deprived of crossed connections: the lamprey hemicord. J. Neurosci. 25, 923–935 (2005)
Grillner, S., Kozlov, A., Dario, P., Stefanini, C., Menciassi, A., Lansner, A., Hellgren Kotaleski, J.: Modeling a vertebrate motor system: pattern generation, steering and control of body orientation. Prog. Brain. Res. 165, 221–234 (2007)
Mullins, O.J., Hackett, J.T., Friesen, W.O.: Modulation of leech swim duration by caudal ganglia. J. Neurophysiol. doi: 10:1152/jn.00507.2010 (2010)
Nusbaum, M.P., Kristan, W.B., Jr.: Swim initiation in the leech by serotonin-containing interneurones, cells 21 and 61. J. Exp. Biol. 122, 277–302 (1986)
Acknowledgement
Funding was provided by NSF grant IOB-0615631.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Friesen, W.O., Mullins, O.J., Xiao, R. et al. Positive feedback loops sustain repeating bursts in neuronal circuits. J Biol Phys 37, 317–345 (2011). https://doi.org/10.1007/s10867-010-9210-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10867-010-9210-8