Abstract
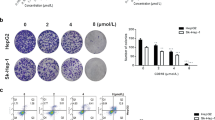
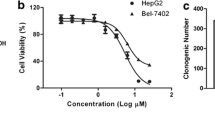
Heat shock protein 90 (Hsp90) is an attractive therapeutic target. Geldanamycin (GA), the first identified Hsp90 inhibitor, exhibited potent antitumor activity, but possessed significant hepatotoxicity. To overcome the hepatotoxicity derived from the quinone structure of GA, a non-quinone GA derivative 17-demethoxy-reblastatin (17-DR) was obtained from a genetically modified strain of Streptomyces hygroscopicus. In the present study, we examined the anticancer effects of 17-DR on human hepatocellular carcinoma (HCC) cell lines HepG2 and SMMC7721, and its underlying mechanisms. The results indicated that 17-DR could concentration-dependently inhibit the proliferation, and decrease the colony formation in HCC cells. It also induced significant apoptosis in HCC cells, which was mediated by mitochondria via a caspase-dependent pathway. The mechanisms involved in 17-DR-induced apoptosis included the downregulation of myeloid cell leukemia-1 (Mcl-1), and upregulation of Bcl-2 antagonist killer 1 (Bak). And the upregulated Bak expression resulted from downregulation of Mcl-1 played an essential role in this process. Taken together, these results indicated that 17-DR possessed potent anticancer effects on HCC cells by inhibiting cell proliferation and inducing apoptosis.




Similar content being viewed by others
References
Adams JM, Cory S (1998) The Bcl-2 protein family: arbiters of cell survival. Science 281:1322–1326
Cervello M, Mccubrey JA, Cusimano A, Lampiasi N, Azzolina A, Montalto G (2012) Targeted therapy for hepatocellular carcinoma: novel agents on the horizon. Oncotarget 3:236–260
Chen D, Shen A, Li J, Shi F, Chen W, Ren J et al (2014) Discovery of potent N-(isoxazol-5-yl)amides as HSP90 inhibitors. Eur J Med Chem 87:765–781
Da Silva VC, Ramos CH (2012) The network interaction of the human cytosolic 90 kDa heat shock protein Hsp90: a target for cancer therapeutics. J Proteomics 75:2790–2802
Dean E, Greystoke A, Ranson M, Dive C (2012) Biomarkers of cell death applicable to early clinical trials. Exp Cell Res 318:1252–1259
Flores A, Marrero JA (2014) Emerging trends in hepatocellular carcinoma: focus on diagnosis and therapeutics. Clin Med Insights Oncol 8:71–76
Gauthier A, Ho M (2013) Role of sorafenib in the treatment of advanced hepatocellular carcinoma: an update. Hepatol Res 43:147–154
Green DR (2005) Apoptotic pathways: ten minutes to dead. Cell 121:671–674
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100:57–70
Kamal A, Thao L, Sensintaffar J, Zhang L, Boehm MF, Fritz LC, Burrows FJ (2003) A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature 425:407–410
Kamal A, Boehm MF, Burrows FJ (2004) Therapeutic and diagnostic implications of Hsp90 activation. Trends Mol Med 10:283–290
Kim T, Keum G, Pae AN (2013) Discovery and development of heat shock protein 90 inhibitors as anticancer agents: a review of patented potent geldanamycin derivatives. Expert Opin Ther Pat 23:919–943
Leanza L, Henry B, Sassi N, Zoratti M, Chandy KG, Gulbins E, Szabo I (2012) Inhibitors of mitochondrial Kv1.3 channels induce Bax/Bak-independent death of cancer cells. EMBO Mol Med 4:577–593
Liu Q, Wang HG (2012) Anti-cancer drug discovery and development: Bcl-2 family small molecule inhibitors. Commun Integr Biol 5:557–565
Martinou JC, Green DR (2001) Breaking the mitochondrial barrier. Nat Rev Mol Cell Biol 2:63–67
Mohana-Kumaran N, Hill DS, Allen JD, Haass NK (2014) Targeting the intrinsic apoptosis pathway as a strategy for melanoma therapy. Pigment Cell Melanoma Res 27:525–539
Neckers L, Mimnaugh E, Schulte TW (1999) Hsp90 as an anti-cancer target. Drug Resist Updat 2:165–172
Pearce AF, Lyles DS (2009) Vesicular stomatitis virus induces apoptosis primarily through Bak rather than Bax by inactivating Mcl-1 and Bcl-XL. J Virol 83:9102–9112
Quinn BA, Dash R, Azab B, Sarkar S, Das SK, Kumar S et al (2011) Targeting Mcl-1 for the therapy of cancer. Expert Opin Investig Drugs 20:1397–1411
Shimazu T, Degenhardt K, Nur EKA, Zhang J, Yoshida T, Zhang Y et al (2007) NBK/BIK antagonizes MCL-1 and BCL-XL and activates BAK-mediated apoptosis in response to protein synthesis inhibition. Genes Dev 21:929–941
Shin JC, Na Z, Lee DH, Kim WC, Lee K, Shen YM et al (2008) Characterization of tailoring genes involved in the modification of geldanamycin polyketide in Streptomyces hygroscopicus JCM4427. J Microbiol Biotechnol 18:1101–1108
Supko JG, Hickman RL, Grever MR, Malspeis L (1995) Preclinical pharmacologic evaluation of geldanamycin as an antitumor agent. Cancer Chemother Pharmacol 36:305–315
Taipale M, Jarosz DF, Lindquist S (2010) HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol 11:515–528
Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM (1994) Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci U S A 91:8324–8328
Wu CZ, Jang JH, Ahn JS, Hong YS (2012) New geldanamycin analogs from Streptomyces hygroscopicus. J Microbiol Biotechnol 22:1478–1481
Zhang H, Burrows F (2004) Targeting multiple signal transduction pathways through inhibition of Hsp90. J Mol Med (Berl) 82:488–499
Zhang W, Liu S, Liu K, Ji B, Wang Y, Liu Y (2014) Knockout of ADAM10 enhances sorafenib antitumor activity of hepatocellular carcinoma in vitro and in vivo. Oncol Rep 32:1913–1922
Zhao Q, Wu CZ, Lee JK, Zhao SR, Li HM, Huo Q et al (2014) Anticancer effects of the Hsp90 inhibitor 17-demethoxy-reblastatin in human breast cancer MDA-MB-231 cells. J Microbiol Biotechnol 24:914–920
Zheng Y, Gery S, Sun H, Shacham S, Kauffman M, Koeffler HP (2014) KPT-330 inhibitor of XPO1-mediated nuclear export has anti-proliferative activity in hepatocellular carcinoma. Cancer Chemother Pharmacol 74:487–495
Zhuo L, Liu J, Wang B, Gao M, Huang A (2013) Differential miRNA expression profiles in hepatocellular carcinoma cells and drug-resistant sublines. Oncol Rep 29:555–562
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81372899, 81302671), the Natural Science Foundation of Anhui Province (1408085QH162), and the Natural Science Foundation of Bengbu Medical College (BYKY1408ZD).
Conflicts of interest
The authors declare that they have no conflict of interests regarding the publication of this paper.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Surong Zhao and Hongmei Li contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Proliferation of HCC cells inhibited by GA. HepG2 and SMMC7721 cells were treated with different concentrations of GA (2.5, 5, 10, 20, 40 μM) or DMSO for 48 h. Cell viability was measured by MTT assay. **p < 0.01 versus no GA treatment (TIFF 730 kb)
Fig. S2
Effects of GA and 17-DR on the viability of normal liver cells. a L-02 cells were treated with different concentrations of GA (5, 10, 20, 40, 80 μM) or DMSO for 48 h. b L-02 cells were treated with different concentrations of 17-DR (25, 50, 100, 200, 400 μM) or DMSO for 48 h. Cell viability was measured by MTT assay. *p < 0.05 and **p < 0.01 versus no GA or 17-DR treatment. (TIFF 991 kb)
Rights and permissions
About this article
Cite this article
Zhao, S., Li, H., Jiang, C. et al. 17-Demethoxy-reblastatin, an Hsp90 inhibitor, induces mitochondria-mediated apoptosis through downregulation of Mcl-1 in human hepatocellular carcinoma cells. J Bioenerg Biomembr 47, 373–381 (2015). https://doi.org/10.1007/s10863-015-9620-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-015-9620-1