Abstract
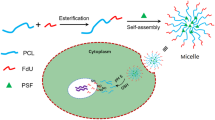
Glucose metabolism of cancer can be used as a strategy to target cancer cells which exhibit altered glycolytic rate. The facilitated glucose transporter (Glut) plays an important role in enhancement glycolytic rate resulting in increased glucose uptake into cancer cells. 18FGD-PET image is an example for using Glut as a targeting to diagnose the high glycolytic rate of tumor. Thus, Glut may be adapted to target cancer cells for drug delivery system. Herein, biodegradation polymeric micelles target cancer cells by Glut was fabricated. The amphiphilic block copolymer of poly(ethylene glycol)-block-poly(ε-caprolactone) (PEG-b-PCL) was synthesized where terminal group of the PEG chain was installed with glucose molecules. The 1H-NMR confirmed the existence of glucose moiety from two distinct peaks (5.2 and 4.7 ppm) of protons at anomeric carbon of glucose. Glucose-PEG-b-PCL spontaneously forms micelles in an aqueous solution. The size and zeta potential were 22 nm and -7 mv, respectively. Glucose-micelles have high stability, and no evidence of cytotoxicity was found after incubation for 7 days. Doxorubicin, used as a fluorescent probe, was loaded into glucose-micelles. The enhanced amount of doxorubicin as a result of glucose-micelles in PC-3, MCF-7 and HepG2 was evaluated by fluorescence microscopy and flow cytometer. Glucose molecules on the surface of micelles increased internalization and enhanced uptake of micelles via bypassing endocytosis pathway. These results show the use of glucose as a targeting ligand on the micelle surface to target cancer cells via Glut.










Similar content being viewed by others
References
Barron CC, Bilan PJ, Tsakiridis T, Tsiani E. Facilitative glucose transporters: implications for cancer detection, prognosis and treatment. Metabolism 2016;65:124–39.
Calvaresi EC, Hergenrother PJ. Glucose conjugation for the specific targeting and treatment of cancer. Chem Sci. 2013;4:2319–33.
Yu J-M, Li W-D, Lu L, Zhou X-Y, Wang D-Y, Li H-M, et al. Preparation and characterization of galactosylated glycol chitosan micelles and its potential use for hepatoma-targeting delivery of doxorubicin. J Mater Sci. 2014;25:691–701.
Xu H, Ma H, Yang P, Zhang X, Wu X, Yin W, et al. Targeted polymer-drug conjugates: current progress and future perspective. Colloids Surf B: Biointerfaces. 2015;136:729–34.
Bowen ML, Chen Z-F, Roos AM, Misri R, Hafeli U, Adam MJ, et al. Long-chain rhenium and technetium glucosamine conjugates. Dalton Trans. 2009;42:9228–36.
Cao J, Cui S, Li S, Du C, Tian J, Wan S, et al. Targeted cancer therapy with a 2-deoxyglucose-based adriamycin complex. Cancer Res. 2013;73:1362–73.
Shan XH, Hu H, Xiong F, Gu N, Geng XD, Zhu W, et al. Targeting Glut1-overexpressing MDA-MB-231 cells with 2-deoxy-d-g1ucose modified SPIOs. Eur J Radiol. 2012;81:95–9.
Jiang X, Xin H, Gu J, Du F, Feng C, Xie Y, et al. Enhanced antitumor efficacy by d-glucosamine-functionalized and paclitaxel-loaded poly(ethylene glycol)-co-poly(trimethylene carbonate) polymer nanoparticles. J Pharm Sci. 2014;103:1487–96.
Venturelli L, Nappini S, Bulfoni M, Gianfranceschi G, Dal Zilio S, Coceano G. Glucose is a key driver for GLUT1-mediated nanoparticles internalization in breast cancer cells. Scientific Reports. 2016;6:21629.
Niu J, Wang A, Ke Z, Zheng Z. Glucose transporter and folic acid receptor-mediated Pluronic P105 polymeric micelles loaded with doxorubicin for brain tumor treating. J Drug Target. 2014;22:712–23.
Jiang X, Xin H, Ren Q, Gu J, Zhu L, Du F, et al. Nanoparticles of 2-deoxy-d-glucose functionalized poly(ethylene glycol)-co-poly(trimethylene carbonate) for dual-targeted drug delivery in glioma treatment. Biomaterials. 2014;35:518–29.
Thasneem YM, Sajeesh S, Sharma CP. Glucosylated polymeric nanoparticles: a sweetened approach against blood compatibility paradox. Colloids Surf B: Biointerfaces. 2013;108:337–44.
Motiei M, Dreifuss T, Betzer O, Panet H, Popovtzer A, Santana J, et al. Differentiating between cancer and inflammation: a metabolic-based method for functional computed tomography imaging. ACS Nano 2016;10:3469–77.
Nittayacharn P, Nasongkla N. Development of self-forming doxorubicin-loaded polymeric depots as an injectable drug delivery system for liver cancer chemotherapy. J Mater Sci. 2017;28:101.
Eawsakul K, Chinavinijkul P, Saeeng R, Chairoungdua A, Tuchinda P, Nasongkla N. Preparation and characterizations of RSPP050-loaded polymeric micelles using poly(ethylene glycol)-b-poly(ε-caprolactone) and poly(ethylene glycol)-b-poly(D,L-lactide). Chem Pharm Bull. 2017;65:530–7.
Tambunlertchai S, Srisang S, Nasongkla N. Development of antimicrobial coating by later-by-layer dip coating of chlorhexidine-loaded micelles. J Mater Sci. 2017;28:90.
Nasongkla N, Shuai X, Ai H, Weinberg BD, Pink J, Boothman DA, et al. cRGD-functionalized polymer micelles for targeted doxorubicin delivery. Angew Chem. 2004;43:6323–7.
Puntawee S, Theerasilp M, Reabroi S, Saeeng R, Piyachaturawat P, Chairoungdua A, et al. Solubility enhancement and in vitro evaluation of PEG- b -PLA micelles as nanocarrier of semi-synthetic andrographolide analogue for cholangiocarcinoma chemotherapy. Pharm Dev Technol. 2016;21:437–44.
Theerasilp M, Chalermpanapun P, Ponlamuangdee K, Sukvanitvichai D, Nasongkla N. Imidazole-modified deferasirox encapsulated polymeric micelles as pH-responsive iron-chelating nanocarrier for cancer chemotherapy. RSC Adv. 2017;7:11158–69.
Caster JM, Patel AN, Zhang T, Wang A. Investigational nanomedicines in 2016: a review of nanotherapeutics currently undergoing clinical trials. Wiley Interdiscip Rev Nanomed Nanobiotech. 2017;9:e1416.
Nakamura T, Nagasaki Y, Kataoka K. Synthesis of heterobifunctional poly(ethylene glycol) with a reducing monosaccharide residue at one end. Bioconjug Chem. 1998;9:300–3.
Yasugi K, Nakamura T, Nagasaki Y, Kato M, Kataoka K. Sugar-installed polymer micelles: synthesis and micellization of poly(ethylene glycol)-poly(D,L-lactide) block copolymers having sugar groups at the PEG chain end. Macromolecules 1999;32:8024–32.
Nagasaki Y, Yasugi K, Yamamoto Y, Harada A, Kataoka K. Sugar-installed block copolymer micelles: their preparation and specific interaction with lectin molecules. Biomacromolecules 2001;2:1067–70.
Yoon JJ, Nam YS, Kim JH, Park TG. Surface immobilization of galactose onto aliphatic biodegradable polymers for hepatocyte culture. Biotechnol Bioeng. 2002;78:1–10.
Rieger J, Stoffelbach F, Cui D, Imberty A, Lameignere E, Putaux JL, et al. Mannosylated poly(ethylene oxide)-b-poly(epsilon-caprolactone) diblock copolymers: synthesis, characterization, and interaction with a bacterial lectin. Biomacromolecules 2007;8:2717–25.
Yang R, Meng F, Ma S, Huang F, Liu H, Zhong Z. Galactose-decorated cross-linked biodegradable poly(ethylene glycol)-b-poly(ε-caprolactone) block copolymer micelles for enhanced hepatoma-targeting delivery of paclitaxel. Biomacromolecules 2011;12:3047–55.
Wang YC, Liu XQ, Sun TM, Xiong MH, Wang J. Functionalized micelles from block copolymer of polyphosphoester and poly(ε-caprolactone) for receptor-mediated drug delivery. J Control Release. 2008;128:32–40.
Nasongkla N, Bey E, Ren J, Ai H, Khemtong C, Guthi JS, et al. Multifunctional polymeric micelles as cancer-targeted, MRI-ultrasensitive drug delivery systems. Nano Lett. 2006;6:2427–30.
Alexandridis P, Holzwarth JF, Hatton TA. Micellization of poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) triblock copolymers in aqueous solutions: thermodynamics of copolymer association. Macromolecules 1994;27:2414–25.
Theerasilp M, Nasongkla N. Comparative studies of poly(ε-caprolactone) and poly(D,L-lactide) as core materials of polymeric micelles. J Microencapsul. 2013;30:390–7.
Narayanan K, Erathodiyil N, Gopalan B, Chong S, Wan ACA, Ying JY. Targeting Warburg effect in cancers with pegylated glucose. Adv Healthc Mater. 2016;5:696–701.
Liu M, Huang G, Cong Y, Tong G, Lin Z, Yin Y. et al. The preparation and characterization of micelles from poly(y-glutamic acid)-graft-poly(l-lactide) and the cellular uptake thereof. J Mater Sci. 2015;26:187.
Wenger RH, Camenisch G, Desbaillets I, Chilov D, Gassmann M. Up-regulation of hypoxia-inducible factor-1α is not sufficient for hypoxic/anoxic p53 induction. Cancer Res. 1998;58:5678–80.
Amann T, Maegdefrau U, Hartmann A, Agaimy A, Marienhagen J, Weiss TS, et al. GLUT1 expression is increased in hepatocellular carcinoma and promotes tumorigenesis. Am J Pathol. 2009;174:1544–52.
Zamora-León SP, Golde DW, Concha II, Rivas CI, Delgado-López F, Baselga J, et al. Expression of the fructose transporter GLUT5 in human breast cancer. Proc Natl Acad Sci USA. 1996;93:1847–52.
Effert P, Beniers AJ, Tamimi Y, Handt S, Jakse G. Expression of glucose transporter 1 (Glut-1) in cell lines and clinical specimens from human prostate adenocarcinoma. Anticancer Res. 2004;24(5A):3057–64.
Petros RA, Desimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov. 2010;9:615–27.
Tian B, Ding Y, Han J, Zhang J, Han Y. N-acetyl-d-glucosamine decorated polymeric nanoparticles for targeted delivery of doxorubicin: synthesis, characterization and in vitro evaluation. Colloids Surf B: Biointerfaces. 2015;130:246–54.
Zhu H, Zhang S, Ling Y, Meng G, Yang Y, Zhang W. PH-responsive hybrid quantum dots for targeting hypoxic tumor siRNA delivery. J Control Release. 2015;220:529–44.
Guo Y, Zhang Y, Li J, Zheng Y, Lu Y, Jiang X, et al. Cell microenvironment-controlled antitumor drug releasing-nanomicelles for GLUT1-targeting hepatocellular carcinoma therapy. ACS Appl Mater Interfaces. 2015;7:5444–53.
Suriano F, Pratt R, Tan JPK, Wiradharma N, Nelson A, Yang YY, et al. Synthesis of a family of amphiphilic glycopolymers via controlled ring-opening polymerization of functionalized cyclic carbonates and their application in drug delivery. Biomaterials 2010;31:2637–45.
Moros M, Hernáez B, Garet E, Dias JT, Sáez B, Grazú V, et al. Monosaccharides versus PEG-functionalized NPs: influence in the cellular uptake. ACS Nano 2012;6:1565–77.
Mumcuoglu D, Sardan Ekiz M, Gunay G, Tekinay T, Tekinay AB, Guler MO. Cellular internalization of therapeutic oligonucleotides by peptide amphiphile nanofibers and nanospheres. ACS Appl Mater Interfaces. 2016;8:11280–7.
Chen H, Kim S, Li L, Wang S, Park K, Cheng JX. Release of hydrophobic molecules from polymer micelles into cell membranes revealed by Förster resonance energy transfer imaging. Proc Natl Acad Sci USA. 2008;105:6596–601.
Acknowledgements
This research project was supported by Mahidol University. Man Theerasilp was partially supported by the Center for Innovation in Chemistry (PERCH-CIC), Commission on Higher Education, Ministry of Education is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Theerasilp, M., Chalermpanapun, P., Sunintaboon, P. et al. Glucose-installed biodegradable polymeric micelles for cancer-targeted drug delivery system: synthesis, characterization and in vitro evaluation. J Mater Sci: Mater Med 29, 177 (2018). https://doi.org/10.1007/s10856-018-6177-7
Published:
DOI: https://doi.org/10.1007/s10856-018-6177-7