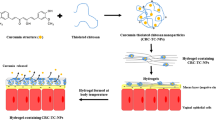
Abstract
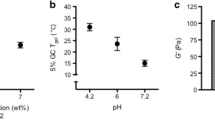
One of the important routes of drug administration for localized delivery of contraceptives and cervical cancer treatment agents is vaginal canal. Due to the low pH of vagina, a pH-responsive drug delivery system was developed. This hydrogel was synthesized based on a mucoadhesive biopolymer, chitosan (CS), that promotes the interaction between the hydrogel and mucosal surface of the vagina, potentially increasing the residence time of the system. This injectable hydrogel was formed via acid-labile Schiff-base linkages between free amine groups and aldehyde functionalities on modified chitosan. A novel approach was taken to add aldehyde functionalities to chitosan using a two-step reaction. Two types of slow and fast degrading hydrogels were prepared and loaded with iron (II) gluconate dihydrate, a non-hormonal spermicide, and doxorubicin hydrochloride, an anti-cancer drug. The release profiles of these drugs at different pH environments were assessed to determine the pH-dependent release mechanism. Mechanical properties, swell-ability and degradation rate of these matrices were studied. The cross-linking density of the hydrogel as well as pH changes played an important role in the characteristic of these hydrogels. The hydrogels degraded faster in lower pH, while the hydrogel with lower cross-linking density showed longer gelation time and faster degradation rate compared to the gel with higher cross-linking density. In vitro cytotoxicity assessment of these hydrogels in 48 h indicated the non-toxic effect of these hydrogels toward mesenchymal stem cells (MSCs) in the test period.










Similar content being viewed by others
References
Hussain A, Ahsan F. The vagina as a route for systemic drug delivery. J Control Release. 2005;103:301–13.
Alexander NJ, Baker E, Kaptein M, Karck U, Miller L, Zampaglione E. Why consider vaginal drug administration? Fertil Steril. 2004;82:1–12.
Machado RM, Palmeira-De-Oliveira A, Martinez-De-Oliveira J, Palmeira-De-Oliveira R. Vaginal films for drug delivery. J Pharm Sci. 2013;102:2069–81.
Ensign LM, Tang BC, Wang Y-Y, Tse TA, Hoen T, Cone R, et al. Mucus-penetrating nanoparticles for vaginal drug delivery protect against herpes simplex virus. Sci. Transl. Med. 2012;4. https://doi.org/10.1126/scitranslmed.3003453.
Yohe ST, Herrera VLM, Colson YL, Grinstaff MW. 3D superhydrophobic electrospun meshes as reinforcement materials for sustained local drug delivery against colorectal cancer cells. J Control Release. 2012;162:92–101.
Wolinsky JB, Colson YL, Grinstaff MW. Local drug delivery strategies for cancer treatment: gels, nanoparticles, polymeric films, rods, and wafers. J Control Release. 2012;159:14–26.
Harwood B, Mishell DR Jr. Contraceptive vaginal rings. Semin Reprod Med. 2001;19:381–90.
Thurman AR, Clark MR, Hurlburt JA, Doncel GF. Intravaginal rings as delivery systems for microbicides and multipurpose prevention technologies. Int J Womens Health. 2013;5:695–708.
Loxley A, Mitchnick M, Okoh O, McConnell J, Goldman L, Morgan C, et al. Ethylene vinyl acetate intravaginal rings for the simultaneous delivery of the antiretroviral UC781 and contraceptive levonorgestrel. Drug Deliv Transl Res. 2011;1:247–55.
Malcolm RK, Forbes CJ, Geer L, Veazey RS, Goldman L, Klasse PJ, et al. Pharmacokinetics and efficacy of a vaginally administered maraviroc gel in rhesus macaques. J Antimicrob Chemother. 2013;68:678–83.
Johnson TJ, Srinivasan P, Albright TH, Watson-Buckheit K, Rabe L, Martin A, et al. Safe and sustained vaginal delivery of pyrimidinedione HIV-1 inhibitors from polyurethane intravaginal rings. J Antimicrob Chemother. 2012;56:1291–9.
Sitruk-Ware R, Nath A, Mishell DR. Contraception technology: past, present and future. Contraception. 2013;87:319–30.
Clifford GM, Smith JS, Plummer M, Muñoz N, Franceschi S. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br J Cancer. 2003;88:63.
Gupta S, Gupta MK. Possible role of nanocarriers in drug delivery against cervical cancer. Nano Rev Exp. 2017;8:1335567.
Ghaemmaghami F, Behtash N, Yarandi F, Moosavi A, Modares M, Toogeh G, et al. First-line chemotherapy with 5-FU and platinum for advanced and recurrent cancer of the cervix: a phase II study. J Obstet Gynaecol. 2003;23:422–5.
Robati M, Holtz D, Dunton CJ. A review of topotecan in combination chemotherapy for advanced cervical cancer. Ther Clin Risk Manag. 2008;4:213–8.
Zeng X, Tao W, Mei L, Huang L, Tan C, Feng S-S. Cholic acid-functionalized nanoparticles of star-shaped PLGA-vitamin E TPGS copolymer for docetaxel delivery to cervical cancer. Biomaterials. 2013;34:6058–67.
Miyamoto T, Takabe Y, Watanabe M, Terasima T. Effectiveness of a sequential combination of bleomycin and mitomycin‐C on an advanced cervical cancer. Cancer. 1978;41:403–14.
Valet P, Senard JM, Devedjian JC, Planat V, Salomon R, Voisin T, et al. Characterization and distribution of alpha 2-adrenergic receptors in the human intestinal mucosa. J Clin Investig. 1993;91:2049–57.
Omura GA, Hubbard J, Hatch K. Chemotherapy of cervix cancer with doxorubicin and cisplatin. A phase I pilot study of the Gynecologic Oncology Group. Am J Clin Oncol. 1985;8:347–9.
Petrou I. Hormonal therapies offer females a targeted acne TX. Dermatol Times. 2007;28:2–S10, S3.
Welling LL. Psychobehavioral effects of hormonal contraceptive use. Evol Psychol. 2013;11:718–42.
Smith JS, Green J, Berrington de Gonzalez A, Appleby P, Peto J, Plummer M, et al. Cervical cancer and use of hormonal contraceptives: a systematic review. Lancet. 2003;361:1159–67.
Moreno V, Bosch FX, Muñoz N, Meijer CJLM, Shah KV, Walboomers JMM, et al. Effect of oral contraceptives on risk of cervical cancer in women with human papillomavirus infection: the IARC multicentric case-control study. Lancet. 2002;359:1085–92.
Han YA, Singh M, Saxena BB. Development of vaginal rings for sustained release of nonhormonal contraceptives and anti-HIV agents. Contraception. 2007;76:132–8.
Saxena BB, Singh M, Gospin RM, Chu CC, Ledger WJ. Efficacy of nonhormonal vaginal contraceptives from a hydrogel delivery system. Contraception. 2004;70:213–9.
Jalalvandi E, Hanton LR, Moratti SC. Schiff-base based hydrogels as degradable platforms for hydrophobic drug delivery. Eur Polym J. 2017;90:13–24.
Liu L, Yao W, Rao Y, Lu X, Gao J. pH-responsive carriers for oral drug delivery: challenges and opportunities of current platforms. Drug Deliv. 2017;24:569–81.
Grassi G, Farra R, Caliceti P, Guarnieri G, Salmaso S, Carenza M, et al. Temperature-sensitive hydrogels. Am J Drug Deliv. 2005;3:239–51.
Anseth KS, Metters AT, Bryant SJ, Martens PJ, Elisseeff JH, Bowman CN. In situ forming degradable networks and their application in tissue engineering and drug delivery. J Control Release. 2002;78:199–209.
Casettari L, Vllasaliu D, Castagnino E, Stolnik S, Howdle S, Illum L. PEGylated chitosan derivatives: synthesis, characterizations and pharmaceutical applications. Progress Polym Sci. 2012;37:659–85.
Deacon MP, Davis SS, White RJ, Nordman H, Carlstedt I, Errington N, et al. Are chitosan–mucin interactions specific to different regions of the stomach? Velocity ultracentrifugation offers a clue. Carbohydr Polym. 1999;38:235–8.
Acarturk F. Mucoadhesive vaginal drug delivery systems. Recent Pat Drug Deliv Formul. 2009;3:193–205.
Rençber S, Karavana SY, Şenyiğit ZA, Eraç B, Limoncu MH, Baloğlu E. Mucoadhesive in situ gel formulation for vaginal delivery of clotrimazole: formulation, preparation, and in vitro/in vivo evaluation. Pharm Dev Technol. 2017;22:551–61.
Clarke MA, Rodriguez AC, Gage JC, Herrero R, Hildesheim A, Wacholder S, et al. A large, population-based study of age-related associations between vaginal pH and human papillomavirus infection. BMC Infect Dis. 2012;12:33.
Krauss-Silva L, Almada-Horta A, Alves MB, Camacho KG, Moreira MEL, Braga A. Basic vaginal pH, bacterial vaginosis and aerobic vaginitis: prevalence in early pregnancy and risk of spontaneous preterm delivery, a prospective study in a low socioeconomic and multiethnic South American population. BMC Pregnancy Childbirth. 2014;14:107.
Kato Y, Onishi H, Machida Y. N-succinyl-chitosan as a drug carrier: water-insoluble and water-soluble conjugates. Biomaterials. 2004;25:907–15.
Aiping Z, Tian C, Lanhua Y, Hao W, Ping L. Synthesis and characterization of N-succinyl-chitosan and its self-assembly of nanospheres. Carbohydr Polym. 2006;66:274–9.
Jalalvandi E, Hanton LR, Moratti SC. Preparation of a pH sensitive hydrogel based on dextran and polyhydrazide for release of 5-flurouracil, an anticancer drug. J Drug Deliv Sci Technol. 2018;44:146–52.
Bae Y, Kataoka K. Intelligent polymeric micelles from functional poly(ethylene glycol)-poly(amino acid) block copolymers. Adv Drug Deliv Rev. 2009;61:768–84.
Xu W, He X, Zhong M, Hu X, Xiao Y. A novel pH-responsive hydrogel based on natural polysaccharides for controlled release of protein drugs. RSC Adv. 2015;5:3157–67.
Jalalvandi E, Cabral J, Hanton LR, Moratti SC. Cyclodextrin-polyhydrazine degradable gels for hydrophobic drug delivery. Mater Sci Eng C. 2016;69:144–53.
Kamoun EA. N-succinyl chitosan–dialdehyde starch hybrid hydrogels for biomedical applications. J Adv Res. 2016;7:69–77.
Horkay F, Han M-H, Han IS, Bang I-S, Magda JJ. Separation of the effects of pH and polymer concentration on the swelling pressure and elastic modulus of a pH-responsive hydrogel. Polymer. 2006;47:7335–8.
Yang B, Li X, Shi S, Kong X, Guo G, Huang M, et al. Preparation and characterization of a novel chitosan scaffold. Carbohydr Polym. 2010;80:860–5.
Yan C, Chen D, Gu J, Hu H, Zhao X, Qiao M. Preparation of N-succinyl-chitosan and their physical-chemical properties as a novel excipient. Yakugaku Zasshi. 2006;126:789–93.
Bashir S, Teo YY, Naeem S, Ramesh S, Ramesh K. pH responsive N-succinyl chitosan/poly(acrylamide-co-acrylic acid) hydrogels and in vitro release of 5-fluorouracil. PLoS ONE. 2017;12:e0179250.
Gipson IK. Mucins of the human endocervix. Front Biosci. 2001;6:D1245–55.
Xiao N, Liang H, Lu J. Degradable and biocompatible aldehyde-functionalized glycopolymer conjugated with doxorubicinvia acid-labile Schiff base linkage for pH-triggered drug release. Soft Matter. 2011;7:10834–40.
Ball C, Krogstad E, Chaowanachan T, Woodrow KA. Drug-eluting fibers for HIV-1 inhibition and contraception. PLoS ONE. 7:e49792.
Hong CY, Lee MF, Lai LJ, Wang CP. Effect of lipid peroxidation on beating frequency of human sperm tail. Andrologia. 1994;26:61–5.
Jones R, Mann T. Lipid peroxides in spermatozoa; formation, role of plasmalogen, and physiological significance. Proc R Soc Lond Ser B Biol Sci. 1976;193:317–33.
Calamera JC, Giovenco P, Quiros MC, Brugo S, Dondero F, Nicholson RF. Effect of lipid peroxidation upon human spermatic adenosinetriphosphate (ATP). Relationship with motility, velocity and linearity of the spermatozoa. Andrologia. 1989;21:48–54.
Acknowledgements
The authors would like to thank Bailey Howe library at UVM and OCEM at Otago University for their resources.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Jalalvandi, E., Shavandi, A. In situ-forming and pH-responsive hydrogel based on chitosan for vaginal delivery of therapeutic agents. J Mater Sci: Mater Med 29, 158 (2018). https://doi.org/10.1007/s10856-018-6166-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-018-6166-x