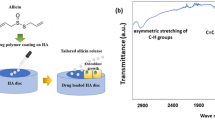
Abstract
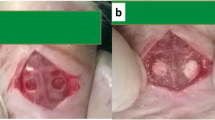
Hydroxyapatite (HA) has been investigated as a delivery system for antimicrobial and antibacterial agents to simultaneously stimulate bone regeneration and prevent infection. Despite evidence supporting the bactericidal efficiency of these HA carriers, few studies have focused on the effect of this association on bone regeneration. In this work, we evaluated the physico-chemical properties of hydroxyapatite microspheres loaded with chlorhexidine (CHX) at two different concentrations, 0.9 and 9.1 \(\upmu {\text{g}}_{\text{CHX}} /{\text{cm}}^{ 2}_{\text{HA}}\), and characterized their effects on in vitro osteoblast viability and bone regeneration. Ultraviolet–visible spectroscopy, scanning and transmission electron microscopy associated with energy-dispersive X-ray spectroscopy and electron energy loss spectroscopy were used to characterize the association of CHX and HA nanoparticles. The high CHX loading dose induced formation of organic CHX plate-like aggregates on the HA surface, whereas a Langmuir film was formed at the low CHX surface concentration. Quantitative evaluation of murine osteoblast viability parameters, including adhesion, mitochondrial activity and membrane integrity of cells exposed to HA/CHX extracts, revealed a cytotoxic effect for both loading concentrations. Histomorphological analysis upon implantation into the dorsal connective tissues and calvaria of rats for 7 and 42 days showed that the high CHX concentration induced the infiltration of inflammatory cells, resulting in retarded bone growth. Despite a strong decrease in in vitro cell viability, the low CHX loading dose did not impair the biocompatibility and osteoconductivity of HA during bone repair. These results indicate that high antimicrobial doses may activate a strong local inflammatory response and disrupt the long-term osteoconductive properties of CHX-HA delivery systems.















Similar content being viewed by others
References
Duyck J, Naert I. Failure of oral implants: aetiology, symptoms and influencing factors. Clin Oral Investig. 1998;2:102–14.
Quirynen M, De Soete M, van Steenberghe D. Infectious risks for oral implants: a review of the literature. Clin Oral Implants Res. 2002;13:1–19.
Zilberman M, Elsner JJ. Antibiotic-eluting medical devices for various applications. J Control Release. 2008;130:202–5.
Arnold RR, Wei HH, Simmons E, Tallury P, Barrow DA, Kalachandra S. Antimicrobial activity and local release characteristics of chlorhexidine diacetate loaded within the dental copolymer matrix, ethylene vinyl acetate. J Biomed Mater Res B Appl Biomater. 2008;86:506–13.
Hanes PJ, Purvis JP. Local anti-infective therapy: pharmacological agents. A systematic review. Ann Periodontol. 2003;8:79–98.
Uskoković V, Desai TA. In vitro analysis of nanoparticulate hydroxyapatite/chitosan composites as potential drug deliveryplatforms for the sustained release of antibiotics in the treatment of osteomyelitis. J Pharm Sci. 2014;103:567–79.
Reise M, Wyrwa R, Müller U, Zylinski M, Völpel A, Schnabelrauch M, Berg A, Jandt KD, Watts DC, Sigusch BW. Release of metronidazole from electrospun poly(l-lactide-co-d/l-lactide) fibers for local periodontitis treatment. Dent Mater. 2012;28:179–88.
Teller M, Gopp U, Neumann HG, Kühn KD. Release of gentamicin from bone regenerative materials: an in vitro study. J Biomed Mater Res B Appl Biomater. 2007;81:23–9.
Garvin K, Feschuk C. Polylactide-polyglycolide antibiotic implants. Clin Orthop Relat Res. 2005;437:105–10.
Bose S, Tarafder S. Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: a review. Acta Biomater. 2012;8:1401–21.
Tautzenberger A, Kovtun A, Ignatius A. Nanoparticles and their potential for application in bone. Int J Nanomed. 2012;7:4545–57.
Habraken WJ, Wolke JG, Jansen JA. Ceramic composites as matrices and scaffolds for drug delivery in tissue engineering. Adv Drug Deliv Rev. 2007;59:234–48.
Rauschmann MA, Wichelhaus TA, Stirnal V, Dingeldein E, Zichner L, Schnettler R, Alt V. Nanocrystalline hydroxyapatite and calcium sulphate as biodegradable composite carrier material for local delivery of antibiotics in bone infections. Biomaterials. 2005;26:2677–84.
Alvarez H, Castro C, Moujir L, Perera A, Delgado A, Soriano I, Evora C, Sánchez E. Efficacy of ciprofloxacin implants in treating experimental osteomyelitis. J Biomed Mater Res B Appl Biomater. 2008;85:93–104.
Dou XC, Zhu XP, Zhou J, Cai HQ, Tang J, Li QL. Minocycline-released hydroxyapatite-gelatin nanocomposite and its cytocompatibility in vitro. Biomed Mater. 2011;6:025002.
Leprêtre S, Chai F, Hornez JC, Vermet G, Neut C, Descamps M, Hildebrand HF, Martel B. Prolonged local antibiotics delivery from hydroxyapatite functionalised with cyclodextrin polymers. Biomaterials. 2009;30:6086–9.
Bhattacharya R, Kundu B, Nandi SK, Basu D. Systematic approach to treat chronic osteomyelitis through localized drug delivery system: bench to bed side. Mater Sci Eng C Mater Biol Appl. 2013;33:3986–93.
Kundu B, Soundrapandian C, Nandi SK, Mukherjee P, Dandapat N, Roy S, Datta BK, Mandal TK, Basu D, Bhattacharya RN. Development of new localized drug delivery system based on ceftriaxone-sulbactam composite drug impregnated porous hydroxyapatite: a systematic approach for in vitro and in vivo animal trial. Pharm Res. 2010;27:1659–76.
Varoni E, Tarce M, Lodi G, Carrassi A. Chlorhexidine (CHX) in dentistry: state of the art. Minerva Stomatol. 2012;61:399–419.
Van Leeuwen MP, Slot DE, Van der Weijden GA. Essential oils compared to chlorhexidine with respect to plaque and parameters of gingival inflammation: a systematic review. J Periodontol. 2011;82:174–94.
Persson RE, Truelove EL, LeResche L, Robinovitch MR. Therapeutic effects of daily or weekly chlorhexidine rinsing on oral health of a geriatric population. Oral Surg Oral Med Oral Pathol. 1991;72:184–91.
McBain AJ, Bartolo RG, Catrenich CE, Charbonneau D, Ledder RG, Gilbert P. Effects of a chlorhexidine gluconate-containing mouthwash on the vitality and antimicrobial susceptibility of in vitro oral bacterial ecosystems. Appl Environ Microbiol. 2003;69:4770–6.
Shen Y, Qian W, Chung C, Olsen I, Haapasalo M. Evaluation of the effect of two chlorhexidine preparations on biofilm bacteria in vitro: a three-dimensional quantitative analysis. J Endod. 2009;35:981–5.
Hita-Iglesias P, Torres-Lagares D, Flores-Ruiz R, Magallanes-Abad N, Basallote-Gonzalez M, Gutierrez-Perez JL. Effectiveness of chlorhexidine gel versus chlorhexidine rinse in reducing alveolar osteitis in mandibular third molar surgery. J Oral Maxillofac Surg. 2008;66:441–5.
Killoy WJ. The use of locally delivered chlorhexidine in the treatment of periodontitis. Clinical results. J Clin Periodontol. 1998;25:953–8.
Flötra L, Gjermo P, Rölla G, Waerhaugh J. Side effects of chlorhexidine mouth washes. Scand J Dent Res. 1971;79:119–25.
Addy M, Prayitno S, Taylor L, Cadogan S. An in vitro study of the role of dietary factors in the aetiology of tooth staining associated with the use of chlorhexidine. J Periodont Res. 1979;14:403–10.
Carpenter GH, Pramanik R, Proctor GB. An in vitro model of chlorhexidine-induced tooth staining. J Periodont Res. 2005;40:225–30.
Van Strydonck DA, Slot DE, Van der Velden U, Van der Weijden F. Effect of a chlorhexidine mouthrinse on plaque, gingival inflammation and staining in gingivitis patients: a systematic review. J Clin Periodontol. 2012;39:1042–55.
De Souza CA, Colombo AP, Souto RM, Silva-Boghossian CM, Granjeiro JM, Alves GG, Rossi AM, Rocha-Leão MH. Adsorption of chlorhexidine on synthetic hydroxyapatite and in vitro biological activity. Colloids Surf B Biointerfaces. 2011;87:310–8.
Ferraz MP, Mateus AY, Sousa JC, Monteiro FJ. Nanohydroxyapatite microspheres as delivery system for antibiotics: release kinetics, antimicrobial activity, and interaction with osteoblasts. J Biomed Mater Res A. 2007;81:994–1004.
ISO 10993-5:2009. Biological evaluation of medical devices - Part 5: Tests for in vitro cytotoxicity.
Taga ML, Granjeiro JM, Cestari TM, Taga R. Healing of critical-size cranial defects in guinea pigs using a bovine bone-derived resorbable membrane. Int J Oral Maxillofac Implants. 2008;23:427–36.
Hallman M, Mordenfeld A, Strandkvist T. Bone replacement following dental trauma prior to implant surgery-present status. Dent Traumatol. 2009;25:2–11.
Lessa FC, Aranha AM, Nogueira I, Giro EM, Hebling J, Costa CA. Toxicity of chlorhexidine on odontoblast-like cells. J Appl Oral Sci. 2010;18:50–8.
Lee TH, Hu CC, Lee SS, Chou MY, Chang YC. Cytotoxicity of chlorhexidine on human osteoblastic cells is related to intracellular glutathione levels. Int Endod J. 2010;43:430–5.
Li YC, Kuan YH, Lee SS, Huang FM, Chang YC. Cytotoxicity and genotoxicity of chlorhexidine on macrophages in vitro. Environ Toxicol. 2014;29:452–8.
Hidalgo E, Dominguez C. Mechanisms underlying chlorhexidine-induced cytotoxicity. Toxicol In Vitro. 2001;15:271–6.
Faria G, Cardoso CR, Larson RE, Silva JS, Rossi MA. Chlorhexidine-induced apoptosis or necrosis in L929 fibroblasts: a role for endoplasmic reticulum stress. Toxicol Appl Pharmacol. 2009;15:256–65.
Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol. 2008;9:231–41.
Zambuzzi WF, Oliveira RC, Pereira FL, Cestari TM, Taga R, Granjeiro JM. Rat subcutaneous tissue response to macrogranular porous anorganic bovine bone graft. Braz Dent J. 2006;17:274–8.
Danesh F, Tootian Z, Jahanbani J, Rabiee M, Fazelipour S, Taghva O, Shabaninia S. Biocompatibility and mineralization activity of fresh or set white mineral trioxide aggregate, biomimetic carbonated apatite, and synthetic hydroxyapatite. J Endod. 2010;36:1036–41.
Calasans-Maia MD, Fernandes GVO, Rossi AM, Dias EP, Almeida GDS, Mitri FF, Granjeiro JM. Effect of hydroxyapatite and zinc-containing hydroxyapatite on osseous repair of critical size defect in the rat calvaria. Key Eng Mater. 2008;361:1273–6.
Conz MB, Granjeiro JM, Soares GDA. Hydroxyapatite crystallinity does not affect the repair of critical size bone defects. J Appl Oral Sci. 2011;19:337–42.
Patel P, Ide M, Coward P, Di Silvio L. The effect of a commercially available chlorhexidine mouthwash product on human osteoblast cells. Eur J Prosthodont Restor Dent. 2006;14:67–72.
Izquierdo-Barba I, Vallet-Regí M, Kupferschmidt N, Terasaki O, Schmidtchen A, Malmsten M. Incorporation of antimicrobial compounds in mesoporous silica film monolith. Biomaterials. 2009;30:5729–36.
Acknowledgments
The authors will receive no benefit of any kind either directly or indirectly. This study was supported in part by National Council for Scientific and Technological Development (CNPq), Coordination of Improvement of Higher Education Personnel (CAPES), Brasilia, Brazil and Foundation for Research Financial Support in the State of Rio de Janeiro (FAPERJ), Rio de Janeiro, Brazil.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soriano-Souza, C.A., Rossi, A.L., Mavropoulos, E. et al. Chlorhexidine-loaded hydroxyapatite microspheres as an antimicrobial delivery system and its effect on in vivo osteo-conductive properties. J Mater Sci: Mater Med 26, 166 (2015). https://doi.org/10.1007/s10856-015-5505-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-015-5505-4