Abstract
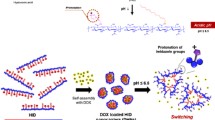
Histidine–hyaluronic acid (His–HA) conjugates were synthesized using hyaluronic acid (HA) as a hydrophilic segment and histidine (His) as hydrophobic segment by 1-ethyl-3(3-dimethylaminopropyl)carbodiimide (EDC) mediated coupling reactions. The structural characteristics of the His–HA conjugates were investigated using 1H NMR. His–HA nanoparticles (HH-NPs) were prepared based on His–HA conjugates, and the characteristics of HH-NPs were investigated using dynamic light scattering, transmission electron microscopy (TEM), scanning electron microscopy (SEM), and fluorescence spectroscopy. The particles were between 342 and 732 nm in size, depending on the degree of substitution (DS) of the His. TEM and SEM images indicated that the morphology of HH-NPs was spherical in shape. The critical aggregation concentrations of HH-NPs ranged from 0.034 to 0.125 mg/ml, which decreased with an increase in the DS of the His. Images of fluorescence microscopy indicate that HH-NPs were taken up by the cancer cell line (MCF-7), and significantly decreased by competition inhibition of free HA. From the cytotoxicity test, it was found that DOX-loaded HH-NPs exhibited similar dose and time-dependent cytotoxicity against MCF-7 cells with free DOX.








Similar content being viewed by others
References
Acharya S, Sahoo SK. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv Drug Deliv Rev. 2011;63:170–83.
Hwang HY, Kim IS, Kwon IC, Kim YH. Tumor targetability and antitumor effect of docetaxel-loaded hydrophobically modified glycol chitosan nanoparticles. J Control Release. 2008;128:23–31.
Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–92.
Choi KY, Chung H, Min KH, Yoon HY, Kim K, Park JH, et al. Self-assembled hyaluronic acid nanoparticles for active tumor targeting. Biomaterials. 2010;31:106–14.
Park K, Lee MY, Kim KS, Hahn SK. Target specific tumor treatment by VEGF siRNA complexed with reducible polyethyleneimine-hyaluronic acid conjugate. Biomaterials. 2010;31:5258–65.
Patil YB, Swaminathan SK, Sadhukha T, Ma L, Panyam J. The use of nanoparticle-mediated targeted gene silencing and drug delivery to overcome tumor drug resistance. Biomaterials. 2010;31:358–65.
Kobayashi T, Ishida T, Okada Y, Ise S, Harashima H, Kiwada H. Effect of transferrin receptor-targeted liposomal doxorubicin in P-glycoprotein-mediated drug resistant tumor cells. Int J Pharm. 2007;329:94–102.
Kirpotin DB, Drummond DC, Shao Y, Shalaby MR, Hong K, Nielsen UB, et al. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res. 2006;66:6732–40.
Licciardi M, Paolino D, Celia C, Giammona G, Cavallaro G, Fresta M. Folate-targeted supramolecular vesicular aggregates based on polyaspartyl-hydrazide copolymers for the selective delivery of antitumoral drugs. Biomaterials. 2010;31:7340–54.
Eliaz RE, Szoka FC Jr. Liposome-encapsulated doxorubicin targeted to CD44: a strategy to kill CD44-overexpressing tumor cells. Cancer Res. 2001;61:2592–601.
Tan YL, Liu CG. Preparation and characterization of self-assembled nanoparticles based on folic acid modified carboxymethyl chitosan. J Mater Sci Mater Med. 2011;22:1213–20.
Gao W, Chan JM, Farokhzad OC. pH-responsive nanoparticles for drug delivery. Mol Pharmacol. 2010;7:1913–20.
Ho VH, Slater NK, Chen R. pH-responsive endosomolytic pseudo-peptides for drug delivery to multicellular spheroids tumour models. Biomaterials. 2011;32:2953–8.
Wang Y, Wu G, Fan Y, Ma J. pH-responsive self-assembly and conformational transition of partially propyl-esterified poly(alpha,beta-l-aspartic acid) as amphiphilic biodegradable polyanion. Colloids Surf B. 2009;68:13–9.
Huh KM, Lee SC, Cho YW, Lee J, Jeong JH, Park K. Hydrotropic polymer micelle system for delivery of paclitaxel. J Control Release. 2005;101:59–68.
Wei H, Zhang XZ, Chen WQ, Cheng SX, Zhuo RX. Self-assembled thermosensitive micelles based on poly(l-lactide-star block-N-isopropylacrylamide) for drug delivery. J Biomed Mater Res A. 2007;83:980–9.
Han G, You CC, Kim BJ, Turingan RS, Forbes NS, Martin CT, et al. Light-regulated release of DNA and its delivery to nuclei by means of photolabile gold nanoparticles. Angew Chem Int Ed Engl. 2006;45:3165–9.
Saslawski O, Weingarten C, Benoit JP, Couvreur P. Magnetically responsive microspheres for the pulsed delivery of insulin. Life Sci. 1988;42:1521–8.
Rivkin I, Cohen K, Koffler J, Melikhov D, Peer D, Margalit R. Paclitaxel-clusters coated with hyaluronan as selective tumor-targeted nanovectors. Biomaterials. 2010;31:7106–14.
He M, Zhao Z, Yin L, Tang C, Yin C. Hyaluronic acid coated poly(butyl cyanoacrylate) nanoparticles as anticancer drug carriers. Int J Pharm. 2009;373:165–73.
Saravanakumar G, Choi KY, Yoon HY, Kim K, Park JH, Kwon IC, et al. Hydrotropic hyaluronic acid conjugates: synthesis, characterization, and implications as a carrier of paclitaxel. Int J Pharm. 2010;394:154–61.
Park W, Kim KS, Bae BC, Kim YH, Na K. Cancer cell specific targeting of nanogels from acetylated hyaluronic acid with low molecular weight. Eur J Pharm Sci. 2010;40:367–75.
Park K, Hong SW, Hur W, Lee MY, Yang JA, Kim SW, et al. Target specific systemic delivery of TGF-beta siRNA/(PEI-SS)-g-HA complex for the treatment of liver cirrhosis. Biomaterials. 2011;32:4951–8.
Gao ZG, Lee DH, Kim DI, Bae YH. Doxorubicin loaded pH-sensitive micelle targeting acidic extracellular pH of human ovarian A2780 tumor in mice. J Drug Target. 2005;13:391–7.
Lee ES, Oh KT, Kim D, Youn YS, Bae YH. Tumor pH-responsive flower-like micelles of poly(l-lactic acid)-b-poly(ethylene glycol)-b-poly(l-histidine). J Control Release. 2007;123:19–26.
Taluja A, Bae YH. Role of a novel excipient poly(ethylene glycol)-b-poly(l-histidine) in retention of physical stability of insulin at aqueous/organic interface. Mol Pharm. 2007;4:561–70.
Li YY, Chen XG, Liu CS, Cha DS, Park HJ, Lee CM. Effect of the molecular mass and degree of substitution of oleoylchitosan on the structure, rheological properties, and formation of nanoparticles. J Agric Food Chem. 2007;55:4842–7.
Park K, Lee GY, Kim YS, Yu M, Park RW, Kim IS, et al. Heparin-deoxycholic acid chemical conjugate as an anticancer drug carrier and its antitumor activity. J Control Release. 2006;114:300–6.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63.
Lee H, Mok H, Lee S, Oh YK, Park TG. Target-specific intracellular delivery of siRNA using degradable hyaluronic acid nanogels. J Control Release. 2007;119:245–52.
Jiang G, Park K, Kim J, Kim KS, Oh EJ, Kang H, et al. Hyaluronic acid-polyethyleneimine conjugate for target specific intracellular delivery of siRNA. Biopolymers. 2008;89:635–42.
Park JS, Han TH, Lee KY, Han SS, Hwang JJ, Moon DH, et al. N-acetyl histidine-conjugated glycol chitosan self-assembled nanoparticles for intracytoplasmic delivery of drugs: endocytosis, exocytosis and drug release. J Control Release. 2006;115:37–45.
Cho HJ, Yoon HY, Koo H, Ko SH, Shim JS, Lee JH, et al. Self-assembled nanoparticles based on hyaluronic acid-ceramide (HA-CE) and Pluronic((R)) for tumor-targeted delivery of docetaxel. Biomaterials. 2011;32:7181–90.
Elizabeth RG. Frechet JMJ pH-Responsive copolymer assemblies for controlled release of doxorubicin. Bioconjug Chem. 2005;16:361–8.
Acknowledgments
This work was supported by the Natural Science Foundation of Shandong Province of China (Grant ZR2009CM071).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, Jl., Liu, Cg., Wang, Xl. et al. Preparation and characterization of nanoparticles based on histidine–hyaluronic acid conjugates as doxorubicin carriers. J Mater Sci: Mater Med 23, 1921–1929 (2012). https://doi.org/10.1007/s10856-012-4665-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-012-4665-8