Abstract
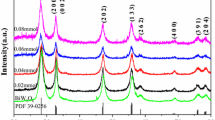
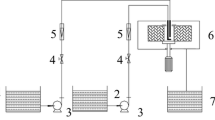
The magnetic photocatalyst Bi2WO6/ZnFe2O4 was loaded on the surface of polyurethane sponge with polyvinyl butyral (PVB) as an immobilizing agent. The as-prepared photocatalyst was characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), energy dispersive spectrometry (EDS) and element mapping. The best operating conditions of the reactor were determined to be 0.10 wt% PVB, mass ratio of sponge to catalyst = 1:1.25, volume ratio of sponge to mixture = 8 g/L and load temperature at 80 °C. The average load rate of the photocatalyst was 56.18%. The photodegradation ratio of tetracycline hydrochloride (TCH, 50 mg/L) was above 98.35% with the load of 5 g/L photocatalyst within 90 min under visible-light irradiation. The best conditions were photocatalyst quantity = 6 g/L, light source intensity = 140000 lux and pollutant concentration = 50 mg/L. The TCH photodegradation ratio reached 91.59%. The addition of PVB increased the fall-off of the photocatalyst and the magnetism of photocatalyst facilitated the recovery. The photocatalyst fall-off rate was 2.53%, and the fall-off part of the powder photocatalyst was adsorbed by magnet, and the recovery rate was 95.13%.








Similar content being viewed by others
References
R. Gothwal, T. Shashidhar, Clean Soil Air Water 43, 479 (2015). doi:10.1002/clen.201300989
I. Yahiaoui, F. Aissani-Benissad, F. Fourcade, A. Amrane, Chem. Eng. J. 221, 418 (2013). doi:10.1016/j.cej.2013.01.091
J.H. Pan, H. Dou, Z. Xiong, C. Xu, J. Ma, X.S. Zhao, J. Mater. Chem. 20, 4512 (2010). doi:10.1039/b925523k
R.L. Pozzo, M.A. Baltanás, A.E. Cassano, Catal Today 39, 219 (1997). doi:10.1016/S0920-5861(97)00103-X
D.S. Kim, Y.S. Park, Chem. Eng. J. 116, 133 (2006). doi:10.1016/j.cej.2005.10.013
X.F. Wang, D.C. Peng, X.L. Hu, Adv. Mater. Res. 472–475, 561 (2012). doi:10.4028/www.scientific.net/AMR.472-475.561
B. Pergolese, M. Muniz-Miranda, A. Bigotto, Chem. Phys. Lett. 438, 290 (2007). doi:10.1016/j.cplett.2007.03.033
G. Balasubramanian, D.D. Dionysiou, M.T. Suidan, V. Subramanian, I. Baudin, J.M. Laîné, J. Mater. Sci. 38, 823 (2003). doi:10.1023/A:1021869200589
P. Rodriguez, V. Meille, S. Pallier, M.A.A. Sawah, Appl. Catal. A Gen. 360, 154 (2009). doi:10.1016/j.apcata.2009.03.013
M. Huang, C. Xu, Z. Wu, Y. Huang, J. Lin, J. Wu, Dyes Pigments 77, 327 (2008). doi:10.1016/j.dyepig.2007.01.026
Y. Iguchi, H. Ichiura, T. Kitaoka, H. Tanaka, Chemosphere 53, 1193 (2003). doi:10.1016/S0045-6535(03)00582-4
G.D. Zhang, Ind. Water Wastewater 46, 8 (2015)
W. Navarrini, M.V. Diamanti, M. Sansotera, et al., Prog. Org. Coat. 74, 794 (2012). doi:10.1016/j.porgcoat.2011.09.023
F. Zhang, S. Zhang, S. Zou, S. Zhong, J. Mater. Sci. Mater. Electron. 27, 12141 (2016). doi:10.1007/s10854-016-5367-7
S. Zhong, N. Song, F. Zhang, Y. Wang, L. Bai, J. Mater. Sci. Mater. Electron. (2017). doi:10.1007/s10854-016-6307-2
S.P. Kamble, S.B.S. And, V.G. Pangarkar, Ind. Eng. Chem. Res. 42, 6705 (2003). doi:10.1021/ie030493r
Acknowledgements
The present work was financially supported by National Natural Science Foundation of China (Grant No. 41402227), also funded by Graduate Innovation Fund of Jilin University (No. 2016099), Jilin Provincial Science and Technology Department (Grant No. 20150204050SF) and Environmental Protection Department of Jilin Province (No. 201419).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, F., Song, N., Zhang, S. et al. Synthesis of sponge-loaded Bi2WO6/ZnFe2O4 magnetic photocatalyst and application in continuous flow photocatalytic reactor. J Mater Sci: Mater Electron 28, 8197–8205 (2017). https://doi.org/10.1007/s10854-017-6530-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-017-6530-5