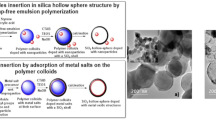
Abstract
The formation of silver and gold nanoparticle-encapsulated hollow carbon spheres (HCS) by twin polymerization is reported. Therefore, silica spheres with different diameters (Aerosil® AS90, d = 20 nm; Aerosil® OX50, d = 40 nm; Stöber particles, d = 200 nm) were coated with the metal carboxylates [AgO2C(CH2OCH2)3H] (1) or [(PPh3)AuO2C(CH2OCH2)3H] (2). Thermal treatment of the as-produced templates gave the respective metal nanoparticle-functionalized systems, which were characterized by powder X-ray diffraction (PXRD) and transmission electron microscopy. The plasmon resonance of the surface-bonded particles was determined by using UV–Vis spectroscopy showing absorptions at 412 nm for silver and 524 nm for gold. The metal nanoparticle-modified templates were then coated with a twin polymer layer as result of the acid-catalyzed twin polymerization of 2,2′-spiro-bi[4H-1,3,2-benzodioxasiline] (3). This coating consists of a phenolic resin and in situ formed silicon dioxide nanoclusters. After carbonization and removal of the SiO2 phase by refluxing with a NaOH solution, the appropriate metal-loaded HCS were obtained. The thus prepared carbon materials were characterized by PXRD, electron microscopy and nitrogen adsorption–desorption isotherms. Mainly micro-porous materials (IUPAC type I) were obtained with a surface area between 910 and 1110 m2/g. These HCS materials were used for the catalytic reduction of methylene blue and 4-nitrophenol proving the accessibility of the incorporated M-NPs. A size-dependent influence of the used carbon hull was found.









Similar content being viewed by others
References
Deshmukh AA, Mhlanga SD, Coville N (2010) Molecular-based design and emerging applications of nanoporous carbon spheres. J Mater Sci Eng R Rep 70:1–28
Hu J, Chen M, Fang X, Wu L (2011) Fabrication and application of inorganic hollow spheres. Chem Soc Rev 40:5472–5491
Schüth F (2014) Encapsulation strategies in energy conversion materials. Chem Mater 26:423–434
Büchel G, Unger KK, Matsumoto A, Tsutsumi K (1998) A novel pathway for synthesis of submicrometer-size solid core/mesoporous shell silica spheres. Adv Mater 10:1036–1038
Yoon SB, Sohn K, Kim JY, Shin C-H, Yu J-S, Hyeon T (2001) Fabrication of carbon capsules with hollow macroporous core/mesoporous shell structures. Adv Mater 14:19–21
Valle-Vigón P, Sevilla M, Fuertes AB (2010) Synthesis of uniform mesoporous carbon capsules by carbonization of organosilica nanospheres. Chem Mater 22:2526–2533
Fang B, Kim M, Yu J-S (2008) Hollow core/mesoporous shell carbon as a highly efficient catalyst support in direct formic acid fuel cell. Appl Catal B Environ 84:100–105
Chai GS, Yoon SB, Kim JH, Yu J-S (2004) Spherical carbon capsules with hollow macroporous core and mesoporous shell structures as a highly efficient catalyst support in the direct methanol fuel cell. Chem Commun 1:2766
Hu FP, Wang Z, Li Y, Li C, Zhang X, Shen PK (2008) Improved performance of Pd electrocatalyst supported on ultrahigh surface area hollow carbon spheres for direct alcohol fuel cells. J Power Sources 177:61–66
Lou XW, Deng D, Lee JY, Archer LA (2008) Preparation of SnO2/carbon composite hollow spheres and their lithium storage properties. Chem Mater 20:6562–6566
Valle-Vigón P, Fuertes AB (2011) Magnetically separable carbon capsules loaded with laccase and their application to dye degradation. RSC Adv 1:1756–1762
Valdes-Solis T, Valle-Vigon P, Sevilla M, Fuertes A (2007) Encapsulation of nanosized catalysts in the hollow core of a mesoporous carbon capsule. J Catal 251:239–243
Lou XW, Li CM, Archer LA (2009) Designed synthesis of coaxial SnO2@carbon hollow nanospheres for highly reversible lithium storage. Adv Mater 21:2536–2539
Zhang W-M, Hu J-S, Guo Y-G, Zheng S-F, Zhong L-S, Song W-G, Wan L-J (2008) Tin-nanoparticles encapsulated in elastic hollow carbon spheres for high-performance anode material in lithium-ion batteries. Adv Mater 20:1160–1165
Kim M, Yoon SB, Sohn K, Kim JY, Shin C-H, Hyeon T, Yu J-S (2003) Synthesis and characterization of spherical carbon and polymer capsules with hollow macroporous core and mesoporous shell structures. Microporous Mesoporous Mater 63:1–9
Zhang Y, Xu S, Luo Y, Pan S, Ding H, Li G (2011) Synthesis of mesoporous carbon capsules encapsulated with magnetite nanoparticles and their application in wastewater treatment. J Mater Chem 21:3664–3671
Sun X, Li Y (2005) Ag@C core/shell structured nanoparticles: controlled synthesis, characterization, and assembly. Langmuir 21:6019–6024
Spange S, Grund S (2009) Nanostructured organic–inorganic composite materials by twin polymerization of hybrid monomers. Adv Mater 21:2111–2116
Spange S, Kempe P, Seifert A, Auer AA, Ecorchard P, Lang H, Falke M, Hietschold M, Pohlers A, Hoyer W, Cox G, Kockrick E, Kaskel S (2009) Nanokomposite mit 0.5 bis 3 nm großen Strukturdomänen durch polymerisation von Silicium-Spiroverbindungen. Angew Chem 121:8403–8408; Angew Chem Int Ed Engl 48:8254–8258
Grund S, Kempe P, Baumann G, Seifert A, Spange S (2007) Zwillingspoly-merisation: ein Weg zur Synthese von Nanokompositen. Angew Chem 119:636–640; Angew Chem Int Ed 46:628–632
Kempe P, Löschner T, Adner D, Spange S (2011) Selective ring opening of 4H-1,3-2-benzodioxasiline twin monomer. New J Chem 35:2735–2739
Kitschke P, Auer AA, Löschner T, Seifert A, Spange S, Rüffer T, Lang H, Mehring M (2014) Microporous carbon and mesoporous silica by use of twin polymerization: an integrated experimental and theoretical approach to precursor reactivity. Chem Plus Chem 79:1009–1023
Löschner T, Mehner A, Grund S, Seifert A, Pohlers A, Lange A, Cox G, Hähnle H.-J, Spange S (2012) Ein modularer Ansatz zur gezielten Herstellung nanostrukturierter Hybridmaterialien: Die Simultane Zwillingspolymerisation. Angew Chem 124(13):3312–3315; Angew Chem Int Ed Engl 51:3258–3261
Böttger-Hiller F, Kempe P, Baumann G, Hietschold M, Schäfer P, Zahn DRT, Petzold A, Thurn-Albrecht T, Spange S (2013) The controlled synthesis of carbon tubes and rods by template-assisted twin polymerization. Adv Mater Sci Eng 2013:1–8
Böttger-Hiller F, Kempe P, Cox G, Panchenko A, Janssen N, Petzold A, Thurn-Albrecht T, Borchardt L, Rose M, Kaskel S, Georgi C, Lang H, Spange S (2013) Zwillingspolymerisation an sphärischen Hart-Templaten—ein Weg zu größeneinstell-baren Kohlenstoffhohlkugeln mit mikro- oder mesoporöser Schale. Angew Chem 25(23):6204–6207; Angew Chem 125:6204–6207
Böttger-Hiller F, Mehner A, Anders S, Kroll L, Cox G, Simon F, Spange S (2012) Sulphur-doped porous carbon from a thiophene-based twin monomer. Chem Commun 48:10568–10570
Brückner J, Thieme S, Böttger-Hiller F, Bauer I, Grossmann HT, Strubel P, Althues H, Spange S, Kaskel S (2014) Carbon-based anodes for lithium-sulfur full cells with high cycle stability. Adv Funct Mater 24:1284–1289
Steffan M, Jakob A, Claus P, Lang H (2009) Silica supported silver nanoparticles from a silver(I) carboxylate: highly active catalyst for regioselective hydrogenation. Catal Commun 10:437–441
Jahn SF, Blaudeck T, Baumann RR, Jakob A, Ecorchard P, Rüffer T, Lang H, Schmidt P (2010) Inkjet printing of conductive silver patterns by using the first aqueous particle-free MOD ink without additional stabilizing ligands. Chem Mater 22:3067–3071
Struppert T, Jakob A, Heft A, Grünler B, Lang H (2010) The use of silver(I)-2-[2-(2-methoxyethoxy)ethoxy]acetate as precursor in the deposition of thin silver layers on float glass by the atmospheric pressure combustion chemical vapor deposition process. Thin Solid Films 518:5741–5744
Tuchscherer A, Schaarschmidt D, Schulze S, Hietschold M, Lang H (2012) Gold nanoparticles generated by thermolysis of “all-in-one” gold(I) carboxylate complexes. Dalton Trans 41:2738–2746
Tuchscherer A, Schaarschmidt D, Schulze S, Hietschold M, Lang H (2011) Simple and efficient: Gold nanoparticles from triphenylphosphane gold(I) carboxylates without addition of any further stabilizing and reducing agent. Inorg Chem Commun 14:676–678
Jahn SF, Jakob A, Blaudeck T, Schmidt P, Lang H, Baumann RR (2010) Inkjet printing of conductive patterns with an aqueous solution of [AgO2C(CH2OCH2)3H] without any additional stabilizing ligands. Thin Solid Films 518:3218–3222
Adner D, Noll J, Schulze S, Hietschold M, Lang H (2016) Asperical silver nanoparticles by thermal decomposition of a single-source-precursor. Inorg Chim Acta 446:19–23
Homola J, Yee SS, Gauglitz G (1999) Surface plasmon resonance sensors: review. Sens Actuators B Chem 54:3–15
Kelly KL, Coronado E, Zhao LL, Schatz GC (2003) The optical properties of metal nanoparticles: the influence of size, shape, and dielectric environment. J Phys Chem B 107:668–677
Buffat P, Borel J-P (1976) Size effect on the melting temperature of gold particles. Phys Rev A 13:2287–2298
Arcidiacono S, Bieri NR, Poulikakos D, Grigoropoulos CP (2004) On the coalescence of gold nanoparticles. Int J Multiph Flow 30:979–994
Lastoskie C, Gubbins KE, Quirke N (1993) Pore size distribution analysis of microporous carbons: a density functional theory approach. J Phys Chem 97:4786–4796
Donohue M, Aranovich GL (1998) Classification of Gibbs adsorption isotherms. Adv Colloid Interface Sci 76–77:137–152
Anderson L, Wittkopp SM, Painter CJ, Liegel JJ, Schreiner R, Bell JA, Shakhashiri BZ (2012) What is happening when the blue bottle bleaches: an investigation of the methylene blue-catalyzed air oxidation of glucose. J Chem Educ 89:1425–1431
Jiang Z-J, Liu C-Y, Sun L-W (2005) Catalytic properties of silver nanoparticles supported on silica spheres. J Phys Chem B 109:1730–1735
Pradhan N, Pal A, Pal T (2001) Catalytic reduction of aromatic nitro compounds by coinage metal nanoparticles. Langmuir 17:1800–1802
Wunder S, Lu Y, Albrecht M, Ballauff M (2011) Catalytic activity of faceted gold nanoparticles studied by a model reaction: evidence for substrate-induced surface restructuring. ACS Catal 1:908–916
Cui J, Gao L, Yan S, Zhang W, Li Y, Gao L (2014) The direct decomposition of nitric oxide over Fe/CNOs (CNOs: carbon nano onions). Synth React Inorg Met Nano Met Chem 45:158–163
Pozun ZD, Rodenbusch SE, Keller E, Tran K, Tang W, Stevenson KJ, Henkelman G (2013) A systematic investigation of p-nitrophenol reduction by bimetallic dendrimer encapsulated nanoparticles. J Phys Chem C Nanomater Interfaces 117:7598–7604
Acknowledgements
We would like to thank T. Jagemann and M. Hiet-schold for SEM imaging of different samples, L. Mertens and M. Mehring for carrying out the powder X-ray diffraction studies and A. Kirillova for preparing the Stöber particles and TEM imaging of the metal nanoparticle-functionalized templates thereof.
Funding
This study was funded by the Deutsche Forschungsgemeinschaft (Grant number: FOR 1497, Organic–Inorganic Nanocomposites by Twin Polymerization).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Schliebe, C., Graske, T., Gemming, T. et al. Metal nanoparticle-loaded porous carbon hollow spheres by twin polymerization. J Mater Sci 52, 12653–12662 (2017). https://doi.org/10.1007/s10853-017-1346-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-1346-5