Abstract
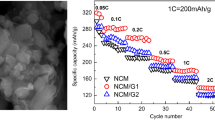
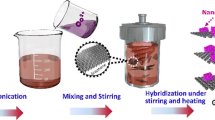
Composite materials consisting of reduced graphene oxide and LiNi0.5Mn1.5O4 were in situ prepared by a simple one-step hydrothermal treating method. The physical property and electrochemical performance of the composite materials were characterized by X-ray diffraction, Raman spectroscopy, scanning electron microscopy, X-ray photoelectron spectroscopy, cyclic voltammetry, charge/discharge testing, and electrochemical impedance spectroscopy. The results demonstrate that the graphene oxide is partially reduced and uniformly in situ anchored on the surface of LiNi0.5Mn1.5O4. As a result, the specific surface area of the composite material dramatically increases from 0.2488 to 8.71 m2 g−1, and the initial specific discharge capacity improves from 125.8 to 140.2 mAh g−1, respectively. Furthermore, the capacity retention maintains 95.8% after 100 cycles, and the electrode polarization has significantly been lessened. At rates of 1, 2, and 5 C, the composite material with 5% reduced graphene oxide can deliver much higher capacities than the pristine LiNi0.5Mn1.5O4. Moreover, AC impedance test results show that the interfacial charge transfer impedance obviously reduced. It is confirmed that the introduction of reduced graphene oxide through hydrothermal treating is effective to enhance the electrochemical performance of the composite material.







Similar content being viewed by others
References
Monaco S, De Giorgio F, Da Col L, Richel M, Arbizzani C, Mastragostino M (2015) Electrochemical performance of LiNi0.5Mn1.5O4 composite electrodes featuring carbons and reduced graphene oxide. J Power Sources 278:733–740
Lee S, Cho Y, Song HK, Lee KT, Cho J (2012) Carbon-coated single-crystal LiMn2O4 nanoparticle clusters as cathode material for high-energy and high-power lithium-ion batteries. Angew Chem 124:8878–8882
Shi J, Wan Z, Fu YQ (2017) Density functional theory analysis of surface structures of spinel LiNi0.5Mn1.5O4 cathode materials. J Mater Sci 52:605–612. doi:10.1007/s10853-016-0357-y
Pieczonka NPW, Liu Z, Lu P, Olson KL, Moote J, Powell BR, Kim JH (2013) Understanding transition-metal dissolution behavior in LiNi0.5Mn1.5O4 high-voltage spinel for lithium ion batteries. J Phys Chem C 117:15947–15957
Kim JH, Pieczonka NPW, Li Z, Wu Y, Harris S, Powell BR (2013) Understanding the capacity fading mechanism in LiNi0.5Mn1.5O4/graphite Li-ion batteries. Electrochim Acta 90:556–562
Mahootcheianasl N, Kim JH, Pieczonka NPW, Liu Z, Kim Y (2013) Multilayer electrolyte cell: a new tool for identifying electrochemical performances of high voltage cathode materials. Electrochem Commun 32:1–4
Liu J, Manthiram A (2009) Understanding the improvement in the electrochemical properties of surface modified 5 V LiMn1.42Ni0.42Co0.16O4 spinel cathodes in lithium-ion cells. Chem Mater 21:1695–1707
Deng JC, Xu YL, Li L, Feng TY, Li L (2016) Microporous LiAlSiO with high ionic conductivity working as a coating material and water adsorbent for LiNiMnO cathode. J Mater Chem A 4:6561–6568
Cho HM, Chen MV, Macrae AC, Meng YS (2015) Effect of surface modification on nano-structured LiNi0.5Mn1.5O4 spinel materials. ACS Appl Mater Interfaces 7:16231–16239
Wu HM, Belharouak I, Abouimrane A, Sun YK, Amine K (2010) Surface modification of LiNi0.5Mn1.5O4 by ZrP2O7 and ZrO2 for lithium-ion batteries. J Power Sources 195:2909–2913
Mo M, Hui KS, Hong X, Guo J, Ye C, Li A, Hu N, Huang Z, Jiang J, Chen H (2014) Improved cycling and rate performance of Sm-doped LiNi0.5Mn1.5O4 cathode materials for 5 V lithium ion batteries. Appl Surf Sci 290:412–418
Xia L, Qiu K, Gao Y, He X, Zhou F (2015) High potential performance of cerium-doped LiNi0.5Co0.2Mn0.3O2, cathode material for li-ion battery. J Mater Sci 50:2914–2920. doi:10.1007/s10853-015-8856-9
Yi TF, Chen B, Zhu YR, Li XY, Zhu RS (2014) Enhanced rate performance of molybdenum-doped spinel LiNi0.5Mn1.5O4 cathode materials for lithium ion battery. J Power Sources 247:778–785
Lee E, Persson KA (2013) First-principles study of the nano-scaling effect on the electrochemical behavior in LiNi0.5Mn1.5O4. Nanotechnology 24:424007
Shaju KM, Bruce PG (2008) Nano- LiNi0.5Mn1.5O4 spinel: a high power electrode for Li-ion batteries. Dalton Trans 63:5471–5475
Myung ST, Komaba S, Kumagai N, Yashiro H, Chung HT, Cho TH (2002) Nano-crystalline LiNi0.5Mn1.5O4 synthesized by emulsion drying method. Electrochim Acta 47:2543–2549
Sun H, Xia B, Liu W, Fang G, Wu J, Wang H, Zhang R, Kaneko S, Zheng J, Wang H, Li D (2015) Significant improvement in performances of LiNi0.5Mn1.5O4 through surface modification with high ordered Al-doped ZnO electro-conductive layer. Appl Surf Sci 331:309–314
Hong D, Guo Y, Wang H, Zhou J, Fang H (2015) Mechanism for improving the cycle performance of LiNi0.5Mn1.5O4 by RuO2 surface modification and increasing discharge cut-off potentials. J Mater Chem A 3:15457–15465
Kang HB, Myung ST, Amine K, Lee SM, Sun YK (2010) Improved electrochemical properties of BiOF-coated 5 V spinel LiNi0.5Mn1.5O4 for rechargeable lithium batteries. J Power Sources 195:2023–2028
Konishi H, Suzuki K, Taminato S, Kim K, Zheng Y, Kim S, Lim J, Hirayama M, Son JY, Cui Y, Kanno R (2014) Effect of surface Li3PO4 coating on LiNi0.5Mn1.5O4 epitaxial thin film electrodes synthesized by pulsed laser deposition. J Power Sources 269:293–298
Kim JW, Kim DH, Oh DY, Lee H, Kim JH, Lee JH, Jung YS (2015) Surface chemistry of LiNi0.5Mn1.5O4 particles coated by Al2O3 using atomic layer deposition for lithium-ion batteries. J Power Sources 274:1254–1262
Li J, Zhang Y, Li J, Wang L, He X, Gao J (2011) AlF3 coating of LiNi0.5Mn1.5O4 for high-performance Li-ion batteries. Ionics 17:671–675
Liu GQ, Wen L, Liu YM (2010) Review spinel LiNi0.5Mn1.5O4 and its derivatives as cathodes for high-voltage Li-ion batteries. J Solid State Electronchem 14:2191–2202
Wang DH, Choi DW, Li J, Yang ZG, Nie ZM, Kou R, Hu DH, Wang CM, Saraf LV, Zhang JG (2009) Self-assembled TiO2-graphene hybrid nanostructures for enhanced Li-ion insertion. ACS Nano 3:907–914
Rao CV, Reddy ALM, Ishikawa Y, Ajayan PM (2011) LiNi1/3Co1/3Mn1/3O2–graphene composite as a promising cathode for lithium-ion batteries. Appl Mater Interfaces 3:2966–2972
Zhou X, Wang F, Zhu Y, Liu Z (2011) Graphene modified LiFePO4 cathode materials for high power lithium ion batteries. J Mater Chem 21:3353–3358
Song B, Lai MO, Liu Z, Liu H, Lu L (2013) Graphene-based surface modification on layered Li-rich cathode for high-performance Li-ion batteries. J Mater Chem A 1:9954–9965
Pyun MH, Park YJ (2015) Graphene/LiMn2O4 nanocomposites for enhanced lithium ion batteries with high rate capability. J Alloys Compd 643:s90–s94
Prabakar SJR, Hwang YH, Lee B, Sohn KS, Pyo M (2013) Graphene-sandwiched LiNi0.5Mn1.5O4 cathode composites for enhanced high voltage performance in Li ion batteries. J Electrochem Soc 160:A832–A837
Tang X, Jan SS, Qian Y, Xia H, Ni J, Savilov SV, Aldoshin SM (2015) Graphene wrapped ordered LiNi0.5Mn1.5O4 nanorods as promising cathode material for lithium-ion batteries. Sci Rep 5:11958
Pei S, Cheng H-M (2012) The reduction of graphene oxide. Carbon 50:3210–3228
Fang X, Ge M, Rong J, Zhou C (2013) Graphene-oxide-coated LiNi0.5Mn1.5O4 as high voltage cathode for lithium ion batteries with high energy density and long cycle life. J Mater Chem A 1:4083–4088
Jia G, Jiao C, Xue W, Zheng S, Wang J (2016) Improvement in electrochemical performance of calcined LiNi0.5Mn1.5O4/GO. Solid State Ionics 292:15–21
Radich JG, Kamat PV (2012) Origin of reduced graphene oxide enhancements in electrochemical energy storage. ACS Catal 2:807–816
Novoselo KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA (2004) Electric field effect in atomically thin carbon films. Science 306:666–669
Mo M, Ye C, Lai K, Huang Z, Zhu L, Ma G, Chen H, Hui KS (2013) Gelatin-assisted synthesis of LiNi0.5Mn1.5O4 cathode material for 5 V lithium rechargeable batteries. Appl Surf Sci 276:635–640
Liu D, Hamel-Paquet J, Trottier J, Barray F, Gariépy V, Hovington P, Guerfi A, Mauger A, Julien CM, Goodenough JB, Zaghi K (2012) Synthesis of pure phase disordered LiMn1.45Cr0.1Ni0.45O4 by a post-annealing method. J Power Sources 217:400–406
Jiang KC, Xin S, Lee JS, Kim J, Xiao XL, Guo YG (2012) Improved kinetics of LiNi1/3Mn1/3Co1/3O2 cathode material through reduced graphene oxide networks. Phys Chem Chem Phys 14:2934–2939
Park JS, Roh KC, Lee JW, Song K, Kim Y, Kang YM (2013) Structurally stabilized LiNi0.5Mn1.5O4 with enhanced electrochemical properties through nitric acid treatment. J Power Sources 230:138–142
Zhou Y, Bao Q, Tang LA, Zhong Y, Loh KP (2009) Hydrothermal dehydration for the “green” reduction of exfoliated graphene oxide to graphene and demonstration of tunable optical limiting properties. Chem Mater 21:2950–2956
Qiao R, Wang Y, Olalde-Velasco P, Li H, Hu Y-S, Yang W (2015) Direct evidence of gradient Mn(II) evolution at charged states in LiNi0.5Mn1.5O4 electrodes with capacity fading. J Power Sources 273:1120–1126
Acknowledgements
Financial support for this work was provided by the Zhejiang University of Science and Technology youth talent cultivation plan, and major science and technology projects of Zhejiang Province (No. 2015C02037).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mo, M., Chen, H., Hong, X. et al. Hydrothermal synthesis of reduced graphene oxide-LiNi0.5Mn1.5O4 composites as 5 V cathode materials for Li-ion batteries. J Mater Sci 52, 2858–2867 (2017). https://doi.org/10.1007/s10853-016-0579-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-016-0579-z