Abstract
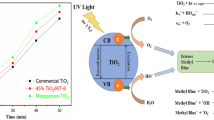
Core–shell-type TiO2@mercaptopropyl-functionalized silica (MPS) particles were prepared for enhanced photocatalytic performance of TiO2. The MPS particles were adopted in the composite-type photocatalyst design so as to enhance the photo-catalytic activity through the chemical interaction between chemically modified substrate particles and organic dye molecules, although they do not have photocatalytic activity. Three organic dyes, rhodamine B, rhodamine 6G, and methyl orange, were selected for testing dye adsorption characteristics and photocatalytic activities of the TiO2@MPS particles, commercial P25, and unsupported TiO2 nanosols. Rhodamine B and rhodamine 6G dyes were adsorbed much more on the TiO2@MPS particles than P25, by factors of 6 and 43, respectively, while methyl orange molecules were adsorbed more readily on P25. Such difference in the dye adsorption characteristics of the two photocatalysts may be related with available functional groups and the dyes. This would lead to enhanced photocatalytic degradation of the dyes because of combined chemical and physical adsorption mechanisms. Toward the two rhodamine dyes, the TiO2@MPS particles show significantly enhanced photocatalytic activity per TiO2 than P25 under both UV and visible light irradiation. Especially under visible irradiation, the degradation rate constants of rhodamine B and rhodamine 6G molecules per TiO2 were higher, 18 and 45 times, respectively, for the TiO2@MPS particles than for P25. Toward methyl orange dye, the degradation rate constant per TiO2 was lower for the TiO2@MPS particles than for P25 under UV irradiation but it was eight times higher under visible irradiation. The TiO2@MPS particles were recyclable and their photocatalytic activity did not change at all at least for three repeated photo-degradation tests.





Similar content being viewed by others
References
Fujishima A, Honda K (1972) Electrochemical photolysis of water at a semiconductor electrode. Nature 238:37–38
Kabra K, Chaudhary R, Sawhney RL (2004) Treatment of hazardous organic and inorganic compounds through aqueous-phase photocatalysis: a review. Ind Eng Chem Res 43:7683–7696
Malato S, Fernández-Ibáñez P, Maldonado MI, Blanco J, Gernjak W (2009) Decontamination and disinfection of water by solar photocatalysis: recent overview and trends. Catal Today 147:1–59
Gaya UI, Abdullah AH (2008) Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: a review of fundamentals, progress and problems. J Photochem Photobiol C 9:1–12
Linsebigler AL, Lu G, Yates JT (1995) Photocatalysis on TiO2 surfaces: principes, mechanisms, and selected results. Chem Rev 95:735–758
Nakata K, Fujishima A (2012) TiO2 photocatalysis: design and applications. J Photochem Photobiol C 13:169–189
Pelaez M, Nolan NT, Pillai SC et al (2012) A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl Catal B 125:331–349
Konstantinou IK, Albanis TA (2004) TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: kinetic and mechanistic investigations a review. Appl Catal B 49:1–14
Turchi CS, Ollis DF (1990) Photocatalytic degradation of organic water contaminants: mechanisms involving hydroxyl radical attack. J Catal 122:178–192
Chen LH, Li XY, Deng Z et al (2013) Hydrothermal and surfactant treatment to enhance the photocatalytic activity of hierarchically meso-macroporous titanias. Catal Today 212:89–97
Kao LH, Hsu TC, Lu HY (2007) Sol–gel synthesis and morphological control of nanocrystalline TiO2 via urea treatment. J Colloid Interface Sci 316:160–167
Ong WJ, Tan LL, Chai SP, Yong ST, Mohamed AR (2014) Facet-dependent photocatalytic properties of TiO2-based composites for energy conversion and environmental remediation. ChemSusChem 7:690–719
Han X, Kuang Q, Jun M, Xie Z, Zheng L (2009) Synthesis of titania nanosheets with a high percentage of exposed (001) facets and related photocatalytic properties. J Am Chem Soc 131:3152–3153
Pang YL, Abdullah AZ (2013) Effect of carbon and nitrogen co-doping on characteristics and sonocatalytic activity of TiO2 nanotues catalyst for degradation of rhodamine B in water. Chem Eng J 214:129–138
Ohno T, Akiyoshi M, Umebayashi T, Asai K, Mitsui T, Matsumura M (2004) Preparation of S-doped TiO2 photocatalysts and their photocatalytic activites under visible light. Appl Catal A 265:115–121
Umebayashi T, Yamaki T, Tanaka S, Asai K (2003) Visible light-induced degradation of methylene blue on S-doped TiO2. Chem Lett 32:330–331
RA, Morikawa T, Ohwaki T, Aoki K, Taga Y (2001) Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 293:269–271
Cong Y, Zhang K, Chen F, Anpo M (2007) Synthesis and characterization of nitrogen-doped TiO2 nanophotocatalyst with high visible light activity. J Phys Chem C 111:6976–6982
Mahlambi MM, Mishra AK, Mishra SB, Krause RW, Mamba BB, Raichur AM (2013) Metal doped nanosized titania used for the photocatalytic degradation of rhodamine B dye under visible-light. J Nanosci Nanotechnol 13:4934–4942
Fukugaichi S, Henmi T, Matsue N (2013) Facile synthesis of TiO2-zeolite composite and its enhanced photocatalytic activity. Catal Lett 143:1255–1259
Liu H, Dong X, Wang X, Sun C, Li J, Zhu Z (2013) A green and direct synthesis of graphene oxide encapsulated TiO2 core/shell structures with enhanced photoactivity. Chem Eng J 230:279–285
Zhang H, Lv X, Li Y, Wang Y, Li J (2010) P25-graphene composite as a high performance photocatalyst. ACS Nano 4:380–386
Muthirulan P, Devi CN, Sundaram MM (2014) TiO2 wrapped graphene as a high performance photocatalyst for acid orange 7 dye degradation uner solar/UV light irradiations. Ceram Int 40:5945–5957
Meng H, Hou W, Xu X, Xu J, Zhang X (2014) TiO2-loaded activated carbon fiber: hydrothermal snythesis, adsorption properties and photocatalytic activity under visible light irradation. Particuology 14:38–43
Zhou W, Pan K, Qu Y et al (2010) Photodegradation of organic contamination in wastewaters by bonding TiO2/single-walled carbon nanotube composites with enhanced photocatalytic activity. Chemosphere 81:555–561
Lim SH, Phonthammachai N, White SSPTJ (2008) Simple route to monodispersed silica–titania core–shell photocatalysts. Langmuir 24:6226–6231
Hu JL, Qian HS, Li JJ, Hu Y, Li ZQ, Yu SH (2013) Synthesis of mesoporous SiO2@TiO2 core–shell nanospheres with enhanced photocatalytic properties. Part Part Syst Charact 30:306–310
Kim SK, Chang H, Cho K et al (2011) Enhanced photocatalytic property of nanoporous TiO2/SiO2 microparticles prepared by aerosol assisted co-assembly of nanoparticles. Mater Lett 65:3330–3332
Anderson C, Bard AJ (1995) An improved photocatalyst of TiO2/SiO2 prepared by a sol–gel synthesis. J Phys Chem 99:9882–9885
Lee JW, Othman MR, Eom Y, Lee TG, Kim WS, Kim J (2008) The effects of sonification and TiO2 deposition on the micro-characteristrics of the thermally treated SiO2/TiO2 spherical core-shell particles for photo-catalysis of methyl orange. Microporous Mesoporous Mater 116:561–568
Xu J, Xiao X, Stepanov AL et al (2013) Efficiency enhancements in Ag nanoparticles-SiO2-TiO2 sandwiched structure via plasmonic effect-enhanced light capturing. Nanoscale Res Lett 8:73
Zhang X, Zhu Y, Yang X et al (2013) Enhanced visible light photocatalytic activity of interlayer-isolatedd triplex Ag@SiO2@TiO2 core–shell nanoparticles. Nanoscale 5:3359–3366
Chen F, Zhao J, Hidaka H (2003) Highly selective deethylation of rhodamine B: adsorption and photooxidation pathways of the dye on the TiO2/SiO2 composite photocatalyst. Int J Photoenergy 5:209–217
Kim JS, Kim SJ, Jung EG, Yun HH, Koo SM (2013) The preparation of titania nano crystals and silica–titania core–shell particles through peptization process. J Ceram Process Res 14:327–331
Wu T, Liu G, Zhao J, Hidaka H, Serpone N (1998) Photoassisted degradation of dye pollutants. V. Self-photosensitized oxidative transformation of rhodamine B under visible light irradiation in aqueous TiO2 dispersions. J Phys Chem B 102:5848–5851
Yu K, Yang S, He H, Sun C, Gu C, Ju Y (2009) Visible light-driven photocatalytic degradation of rhodamine B over NaBiO3: pathways and mechanism. J Phys Chem A 113:10024–10032
Wang Q, Chen C, Zhao D, Ma W, Zhao J (2008) Change of adsorption modes of dyes on fluorinated TIO2 and its effect on photocatalytic degradation of dyes under visible irradiation. Langmuir 24:7338–7345
Sung-Suh HM, Choi JR, Hah HJ, Koo SM, Bae YC (2004) Comparison of Ag deposition effects on the photocatalytic activity of nanoparticulate TiO2 under visible and UV light irradiation. J Photochem Photobiol A 163:37–44
Lubec G (1996) The hydroxyl radical: from chemistry to human disease. J Investig Med 44:324–346
Khodja AA, Sehili T, Pilichowski J-F, Boule P (2001) Photocatalytic degradation of 2-phenylphenol on TiO2 and ZnO in aqueous suspensions. J Photochem Photobiol A 141:231–239
Daneshvar N, Salari D, Khataee AR (2003) Photocatalytic degradation of azo dye acid red 14 in water: investigation of the effect of operational parameters. J Photochem Photobiol, A 157:111–116
Battino R, Rettich TR, Tominaga T (1983) The solubility of oxygen and ozone in liquids. J Phys Chem Ref Data 12:163–178
Acknowledgements
This work was supported by the Human Resources Development Program (NO. 20094020200010) of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) grant funded by the Korea government Ministry of Trade, Industry and Energy and also supported by Nano-Convergence Foundation (www.nanotech2020.org) funded by the Ministry of Science, ICT and Future Planning (MSIP, Korea) & the Ministry of Trade, Industry and Energy(MOTIE, Korea) (Project Name: 1415131476) and Hanyang Science and Technology Scholarship Program.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yun, H.H., Kim, J.S., Kim, E.H. et al. Enhanced photocatalytic activity of TiO2@mercapto-functionalized silica toward colored organic dyes. J Mater Sci 50, 2577–2586 (2015). https://doi.org/10.1007/s10853-015-8823-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-015-8823-5