Abstract
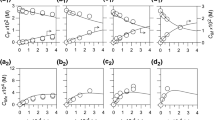
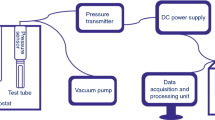
Acetamide and formamide were individually decomposed in a pressurized hot water in a tubular flow reactor at temperatures from 573 to 693 K, and pressure of 23 MPa, residence times up to 500 s, and the initial concentrations of both amides from 0.005 to 0.5 mol/L. The major products were ammonia and acetic acid from decomposition of acetamide, and ammonia and formic acid from that of formamide. Formic acid was further decomposed readily into carbon dioxide. Although the decomposition reactions for both amides were represented acceptably by the first order reaction kinetics, the rate constants increased with increasing the initial sample concentrations due to the autocatalytic effect. Apparently the second order reaction kinetics with respect to the concentration of each amide remained more represented the global decomposition rates, and the rate constants decreased with increasing the initial concentrations. The effects of hydrogen peroxide added on the global decomposition rates and the product yields were not evident: the addition slightly lowered the rates, but the major products were almost the same as those in the absence of hydrogen peroxide at temperatures lower than 653 K. Above 653 K more CO2 was produced.
Similar content being viewed by others
References
P. E. SAVAGE, CHEM. REV. 99 (1999) 603.
S. IMAMURA, IND. ENG. CHEM. RES. 38 (1999) 174.
T. B. BRILL, J. PHYS. CHEM. 104 (2000) 4343.
N. CRAIN, S. TEBBAL, L. LI AND E. F. GLOYNA, Ind. Eng. Chem. Res. 32 (1993) 2259.
J. W. SCHOPPELREI, M. L. KIEKE, X. WANG, M. T. KLEIN AND T. B. BRILL, J. Phys. Chem. 100 (1996) 14343.
S. D. IYER AND M. T. KLEIN, J. Supercrit. Fluids 10 (1997) 191.
M. J. COCERO, E. ALONSO, R. TORIO, D. VALLELADO AND F. FDZ-POLANCO, Ind. Eng. Chem. Res. 39 (2000) 3707.
D. LEE AND E. F. GLOYNA, Environ. Sci. Technol. 26 (1992) 1587.
B. IZZO, C. L. HARRELL AND M. T. KLEIN, AIChE J. 43 (1997) 2048.
B. IZZO, M. T. KLEIN, C. LAMARCA AND N. C. SCRIVER, Ind. Eng. Chem. Res. 38 (1999) 1183.
Y. OSHIMA, B. BIJANTO AND S. KODA, ibid. 40 (2001) 1026.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Okazaki, M., Funazukuri, T. Decomposition of acetamide and formamide in pressurized hot water. J Mater Sci 41, 1517–1521 (2006). https://doi.org/10.1007/s10853-006-4616-1
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10853-006-4616-1