Abstract
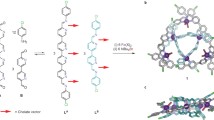
A new method for the synthesis of interlocked compounds utilizing the catalytic activity of macrocyclic phenanthroline–Cu complexes was developed. The macrocyclic phenanthroline–Cu complexes were found to be good catalysts for several coupling reactions, and this catalytic activity was subsequently utilized for the synthesis of [2]rotaxanes and [2]catenanes. By combining the catalytic threading approach with the well-known metal-template method, several rotacatenanes were synthesized. In addition, one-pot synthesis of [3]rotaxanes was achieved in good yield by performing the threading reaction twice in a one-ring component.




















Similar content being viewed by others
References
Sauvage, J.P.: Interlacing molecular threads on transition metals: catenands, catenates, and knots. Acc. Chem. Res. 23(10), 319–327 (1990). doi:10.1021/ar00178a001
Amabilino, D.B., Stoddart, J.F.: Interlocked and intertwined structures and superstructures. Chem. Rev. 95(8), 2725–2828 (1995). doi:10.1021/cr00040a005
Jäger, R., Vögtle, F.: A new synthetic strategy towards molecules with mechanical bonds: nonionic template synthesis of amide-linked catenanes and rotaxanes. Angew. Chem. Int. Ed. 36(9), 930–944 (1997). doi:10.1002/anie.199709301
Nepogodiev, S.A., Stoddart, J.F.: Cyclodextrin-based catenanes and rotaxanes. Chem. Rev. 98(5), 1959–1976 (1998). doi:10.1021/cr970049w
Raymo, F.M., Stoddart, J.F.: Interlocked macromolecules. Chem. Rev. 99(7), 1643–1664 (1999). doi:10.1021/cr970081q
Niu, Z., Gibson, H.W.: Polycatenanes. Chem. Rev. 109(11), 6024–6046 (2009). doi:10.1021/cr900002h
Crowley, J.D., Goldup, S.M., Lee, A.-L., Leigh, D.A., McBurney, R.T.: Active metal template synthesis of rotaxanes, catenanes and molecular shuttles. Chem. Soc. Rev. 38(6), 1530–1541 (2009). doi:10.1039/B804243H
Beves, J.E., Blight, B.A., Campbell, C.J., Leigh, D.A., McBurney, R.T.: Strategies and tactics for the metal-directed synthesis of rotaxanes, knots, catenanes, and higher order links. Angew. Chem. Int. Ed. 50(40), 9260–9327 (2011). doi:10.1002/anie.201007963
Forgan, R.S., Sauvage, J.-P., Stoddart, J.F.: Chemical topology: complex molecular knots, links, and entanglements. Chem. Rev. 111(9), 5434–5464 (2011). doi:10.1021/cr200034u
Neal, E.A., Goldup, S.M.: Chemical consequences of mechanical bonding in catenanes and rotaxanes: isomerism, modification, catalysis and molecular machines for synthesis. Chem. Commun. 50(40), 5128–5142 (2014). doi:10.1039/C3CC47842D
Sauvage, J.-P.: Transition metal-containing rotaxanes and catenanes in motion: toward molecular machines and motors. Acc. Chem. Res. 31(10), 611–619 (1998). doi:10.1021/ar960263r
Tian, H., Wang, Q.-C.: Recent progress on switchable rotaxanes. Chem. Soc. Rev. 35(4), 361–374 (2006). doi:10.1039/B512178G
Harrison, I.T., Harrison, S.: Synthesis of a stable complex of a macrocycle and a threaded chain. J. Am. Chem. Soc. 89(22), 5723–5724 (1967). doi:10.1021/ja00998a052
Schill, G.; Zollenkopf, H. Justus Liebigs Ann. Chem. 721, 53–74 (1969)
Hiratani, K., Suga, J.-I., Nagawa, Y., Houjou, H., Tokuhisa, H., Numata, M., Watanabe, K.: A new synthetic method for rotaxanes via tandem Claisen rearrangement, diesterification, and aminolysis. Tetrahedron Lett. 43(33), 5747–5750 (2002). doi:10.1016/S0040-4039(02)01201-7
Kawai, H., Umehara, T., Fujiwara, K., Tsuji, T., Suzuki, T.: Dynamic covalently bonded rotaxanes cross-linked by imine bonds between the axle and ring: inverse temperature dependence of subunit mobility. Angew. Chem. 118(26), 4387–4392 (2006). doi:10.1002/ange.200600750
Hirose, K., Nishihara, K., Harada, N., Nakamura, Y., Masuda, D., Araki, M., Tobe, Y.: Highly selective and high-yielding rotaxane synthesis via aminolysis of prerotaxanes consisting of a ring component and a stopper unit. Org. Lett. 9(16), 2969–2972 (2007). doi:10.1021/ol070999w
Ashton, P.R., Baxter, I., Fyfe, M.C.T., Raymo, F.M., Spencer, N., Stoddart, J.F., White, A.J.P., Williams, D.J.: Rotaxane or pseudorotaxane? That is the question! J. Am. Chem. Soc. 120(10), 2297–2307 (1998). doi:10.1021/ja9731276
Hübner, G.M., Gläser, J., Seel, C., Vögtle, F.: High-yielding rotaxane synthesis with an anion template. Angew. Chem. Int. Ed. 38(3), 383–386 (1999). doi:10.1002/(SICI)1521-3773(19990201)38:3<383:AID-ANIE383>3.0.CO;2-H
Takata, T., Kawasaki, H., Asai, S., Furusho, Y., Kihara, N.: Conjugate addition-approach to end-capping of pseudorotaxanes for rotaxane synthesis. Chem. Lett. 28(3), 223–224 (1999). doi:10.1246/cl.1999.223
Johnston, A.G., Leigh, D.A., Murphy, A., Smart, J.P., Deegan, M.D.: The synthesis and solubilization of amide macrocycles via rotaxane formation. J. Am. Chem. Soc. 118(43), 10662–10663 (1996). doi:10.1021/ja962046r
Vögtle, F., Händel, M., Meier, S., Ottens-Hildebrandt, S., Ott, F., Schmidt, T.: Template synthesis of the first amide-based rotaxanes. Liebigs Annalen 1995(5), 739–743 (1995). doi:10.1002/jlac.1995199505109
Hunter, C.A., Low, C.M.R., Packer, M.J., Spey, S.E., Vinter, J.G., Vysotsky, M.O., Zonta, C.: Noncovalent assembly of [2]rotaxane architectures. Angew. Chem. Int. Ed. 40(14), 2678–2682 (2001). doi:10.1002/1521-3773(20010716)40:14<2678:AID-ANIE2678>3.0.CO;2-U
Loeb, S.J., Wisner, J.A.: [3]Rotaxanes employing multiple 1,2-bis(pyridinium) ethane binding sites and dibenzo-24-crown-8 ethers. Chem. Commun. 10, 845–846 (2000). doi:10.1039/B001018I
Davidson, G.J.E., Loeb, S.J., Parekh, N.A., Wisner, J.A.: Zwitterionic [2]rotaxanes utilising anionic transition metal stoppers. J. Chem. Soc., Dalton Trans. 21, 3135–3136 (2001). doi:10.1039/B107257A
Tokunaga, Y., Akasaka, K., Hashimoto, N., Yamanaka, S., Hisada, K., Shimomura, Y., Kakuchi, S.: Using photoresponsive end-closing and end-opening reactions for the synthesis and disassembly of [2]rotaxanes: implications for dynamic covalent chemistry. J. Org. Chem. 74(6), 2374–2379 (2009). doi:10.1021/jo8025143
Iwamoto, H., Yawata, Y., Fukazawa, Y., Haino, T.: Tether-assisted synthesis of [3]rotaxane by Olefin metathesis. Chem. Lett. 39(1), 24–25 (2010). doi:10.1246/cl.2010.24
Yu, G., Suzaki, Y., Abe, T., Osakada, K.: Organometallic rotaxanes with a triazole group in the axle component and their behavior as ligands of ptii complexes. Chemistry 7(1), 207–213 (2012). doi:10.1002/asia.201100617
Wu, C., Lecavalier, P.R., Shen, Y.X., Gibson, H.W.: Synthesis of a rotaxane via the template method. Chem. Mater. 3(4), 569–572 (1991). doi:10.1021/cm00016a002
Chambron, J.-C., Heitz, V., Sauvage, J.-P.: A rotaxane with two rigidly held porphyrins as stoppers. J. Chem. Soc. Chem. Commun. 16, 1131–1133 (1992). doi:10.1039/C39920001131
Furusho, Y., Matsuyama, T., Takata, T., Moriuchi, T., Hirao, T.: Synthesis of novel interlocked systems utilizing a palladium complex with 2,6-pyridinedicarboxamide-based tridentate macrocyclic ligand. Tetrahedron Lett. 45(52), 9593–9597 (2004). doi:10.1016/j.tetlet.2004.10.152
Ogino, H.: Relatively high-yield syntheses of rotaxanes. Syntheses and properties of compounds consisting of cyclodextrins threaded by.alpha.,omega.-diaminoalkanes coordinated to cobalt(III) complexes. J. Am. Chem. Soc. 103(5), 1303–1304 (1981). doi:10.1021/ja00395a091
Harada, A., Li, J., Kamachi, M.: The molecular necklace: a rotaxane containing many threaded α-cyclodextrins. Nature 356(6367), 325–327 (1992)
Wenz, G., Keller, B.: Threading Cyclodextrin Rings on Polymer Chains. Angew. Chem. Int. Ed. 31(2), 197–199 (1992). doi:10.1002/anie.199201971
Anderson, S., Claridge, T.D.W., Anderson, H.L.: Azo-dye rotaxanes. Angew. Chem. Int. Ed. 36(12), 1310–1313 (1997). doi:10.1002/anie.199713101
Nakashima, N., Kawabuchi, A., Murakami, H.: Design and synthesis of cyclodextrin-based rotaxanes and polyrotaxanes. J. Incl. Phenom. Mol. Recognit. Chem. 32(2–3), 363–373 (1998). doi:10.1023/A:1008084015958
Terao, J., Tanaka, Y., Tsuda, S., Kambe, N., Taniguchi, M., Kawai, T., Saeki, A., Seki, S.: Insulated molecular wire with highly conductive π-conjugated polymer core. J. Am. Chem. Soc. 131(50), 18046–18047 (2009). doi:10.1021/ja908783f
Strutt, N.L., Forgan, R.S., Spruell, J.M., Botros, Y.Y., Stoddart, J.F.: Monofunctionalized pillar[5]arene as a host for alkanediamines. J. Am. Chem. Soc. 133(15), 5668–5671 (2011). doi:10.1021/ja111418j
Ogoshi, T., Aoki, T., Shiga, R., Iizuka, R., Ueda, S., Demachi, K., Yamafuji, D., Kayama, H., Yamagishi, T.-A.: Cyclic host liquids for facile and high-yield synthesis of [2]rotaxanes. J. Am. Chem. Soc. 134(50), 20322–20325 (2012). doi:10.1021/ja310757p
Ashton, P.R., Grognuz, M., Slawin, A.M.Z.: Fraser Stoddart, J., Williams, D.J.: The template-directed synthesis of a [2]rotaxane. Tetrahedron Lett. 32(43), 6235–6238 (1991). doi:10.1016/0040-4039(91)80797-A
Hancock, L.M., Beer, P.D.: Chloride recognition in aqueous media by a rotaxane prepared via a new synthetic pathway. Chem. Eur. J. 15(1), 42–44 (2009). doi:10.1002/chem.200802029
Dietrich-Buchecker, C.O., Sauvage, J.P., Kintzinger, J.P.: New family of molecules: the metallo-catenanes. Tetrahedron Lett. 24(46), 5095–5098 (1983). doi:10.1016/S0040-4039(00)94050-4
Aucagne, V., Hänni, K.D., Leigh, D.A., Lusby, P.J., Walker, D.B.: Catalytic “click” rotaxanes: a substoichiometric metal-template pathway to mechanically interlocked architectures. J. Am. Chem. Soc. 128(7), 2186–2187 (2006). doi:10.1021/ja056903f
Saito, S., Takahashi, E., Nakazono, K.: Synthesis of [2]rotaxanes by the catalytic reactions of a macrocyclic copper complex. Org. Lett. 8(22), 5133–5136 (2006). doi:10.1021/ol062247s
Bates, C.G., Gujadhur, R.K., Venkataraman, D.: A general method for the formation of aryl–sulfur bonds using copper(I) catalysts. Org. Lett. 4(16), 2803–2806 (2002). doi:10.1021/ol0264105
Siemsen, P., Livingston, R.C., Diederich, F.: Acetylenic coupling: a powerful tool in molecular construction. Angew. Chem. Int. Ed. 39(15), 2632–2657 (2000). doi:10.1002/1521-3773(20000804)39:15<2632:AID-ANIE2632>3.0.CO;2-F
Hay, A.S.: Oxidative coupling of acetylenes. II. J. Org. Chem. 27(9), 3320–3321 (1962). doi:10.1021/jo01056a511
Bates, C.G., Saejueng, P., Venkataraman, D.: Copper-catalyzed synthesis of 1,3-enynes. Org. Lett. 6(9), 1441–1444 (2004). doi:10.1021/ol049706e
Monnier, F., Turtaut, F., Duroure, L., Taillefer, M.: Copper-catalyzed sonogashira-type reactions under mild palladium-free conditions. Org. Lett. 10(15), 3203–3206 (2008). doi:10.1021/ol801025u
Santandrea, J., Bédard, A.-C., Collins, S.K.: Cu(I)-catalyzed macrocyclic sonogashira-type cross-coupling. Org. Lett. 16(15), 3892–3895 (2014). doi:10.1021/ol501898b
Saito, S., Nakazono, K., Takahashi, E.: Template synthesis of [2]rotaxanes with large ring components and tris(biphenyl) methyl group as the blocking group. The relationship between the ring size and the stability of the rotaxanes. J. Org. Chem. 71(19), 7477–7480 (2006)
Saito, S., Takahashi, E., Wakatsuki, K., Inoue, K., Orikasa, T., Sakai, K., Yamasaki, R., Mutoh, Y., Kasama, T.: Synthesis of large [2]rotaxanes. The relationship between the size of the blocking group and the stability of the rotaxane. J. Org. Chem. 78(8), 3553–3560 (2013). doi:10.1021/jo302800t
Weisbach, N., Baranova, Z., Gauthier, S., Reibenspies, J.H., Gladysz, J.A.: A new type of insulated molecular wire: a rotaxane derived from a metal-capped conjugated tetrayne. Chem. Commun. 48(61), 7562–7564 (2012). doi:10.1039/C2CC33321J
Movsisyan, L.D., Kondratuk, D.V., Franz, M., Thompson, A.L., Tykwinski, R.R., Anderson, H.L.: Synthesis of polyyne rotaxanes. Org. Lett. 14(13), 3424–3426 (2012). doi:10.1021/ol301392t
Movsisyan, L.D., Peeks, M.D., Greetham, G.M., Towrie, M., Thompson, A.L., Parker, A.W., Anderson, H.L.: Photophysics of threaded sp-carbon chains: the polyyne is a sink for singlet and triplet excitation. J. Am. Chem. Soc. 136(52), 17996–18008 (2014). doi:10.1021/ja510663z
Ugajin, K., Takahashi, E., Yamasaki, R., Mutoh, Y., Kasama, T., Saito, S.: Synthesis of [2]rotaxanes by the copper-mediated threading reactions of aryl iodides with alkynes. Org. Lett. 15(11), 2684–2687 (2013). doi:10.1021/ol400992p
Megiatto, J.D., Schuster, D.I.: Alternative demetalation method for Cu(I)-phenanthroline-based catenanes and rotaxanes. Org. Lett. 13(7), 1808–1811 (2011). doi:10.1021/ol200304d
Sato, Y., Yamasaki, R., Saito, S.: Synthesis of [2]catenanes by oxidative intramolecular diyne coupling mediated by macrocyclic copper(I) complexes. Angew. Chem. Int. Ed. 48(3), 504–507 (2009). doi:10.1002/anie.200804864
Amabilino, D.B., Ashton, P.R., Bravo, J.A., Raymo, F.M., Stoddart, J.F., White, A.J.P., Williams, D.J.: Molecular meccano, 52 template-directed synthesis of a rotacatenane. Eur. J. Org. Chem. 1999(6), 1295–1302 (1999). doi:10.1002/(SICI)1099-0690(199906)1999:6<1295:AID-EJOC1295>3.0.CO;2-Z
Barin, G., Coskun, A., Friedman, D.C., Olson, M.A., Colvin, M.T., Carmielli, R., Dey, S.K., Bozdemir, O.A., Wasielewski, M.R., Stoddart, J.F.: A multistate switchable [3]rotacatenane. Chem. Eur. J. 17(1), 213–222 (2011). doi:10.1002/chem.201002152
Hayashi, R., Wakatsuki, K., Yamasaki, R., Mutoh, Y., Kasama, T., Saito, S.: Synthesis of rotacatenanes by the combination of Cu-mediated threading reaction and the template method: the dual role of one ligand. Chem. Commun. 50(2), 204–206 (2014). doi:10.1039/C3CC47425A
Yerin, A., Wilks, E.S., Moss, G.P., Harada, A.: Nomenclature for rotaxanes and pseudorotaxanes—(IUPAC recommendations 2008). Pure Appl. Chem. 80(9), 2041–2068 (2008). doi:10.1351/pac200880092041
Klotz, E.J.F., Claridge, T.D.W., Anderson, H.L.: Homo- and hetero-[3]rotaxanes with two π-systems clasped in a single macrocycle. J. Am. Chem. Soc. 128(48), 15374–15375 (2006). doi:10.1021/ja0665139
Prikhod’ko, A.I., Durola, F., Sauvage, J.-P.: Iron(II)-templated synthesis of [3]rotaxanes by passing two threads through the same ring. J. Am. Chem. Soc. 130(2), 448–449 (2007). doi:10.1021/ja078216p
Cheng, H.M., Leigh, D.A., Maffei, F., McGonigal, P.R., Slawin, A.M.Z., Wu, J.: En route to a molecular sheaf: active metal template synthesis of a [3]rotaxane with two axles threaded through one ring. J. Am. Chem. Soc. 133(31), 12298–12303 (2011). doi:10.1021/ja205167e
Yamashita, Y., Mutoh, Y., Yamasaki, R., Kasama, T., Saito, S.: Synthesis of [3]rotaxanes that utilize the catalytic activity of a macrocyclic phenanthroline–Cu complex: remarkable effect of the length of the axle precursor. Chem. Eur. J. 21(5), 2139–2145 (2015). doi:10.1002/chem.201405090
Acknowledgments
The author thanks the Organizing Committee of Host–Guest and Supramolecular Chemistry Society, Japan for giving him the HGCS Japan Award of Excellence 2014 and the opportunity to write this review. The author acknowledges Dr. Ryu Yamasaki, Dr. Yuichiro Mutoh, and all collaborators for their significant contribution. The author thanks Dr. Takeshi Kasama (Tokyo Medical and Dental University) for performing MALDI-TOF MS analysis. This work was supported by JSPS KAKENHI Grant Number 26410125 and 21655017, Yamada Science Foundation, and the UBE Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is selected for “HGCS Japan Award of Excellence 2014”.
Rights and permissions
About this article
Cite this article
Saito, S. Synthesis of interlocked compounds utilizing the catalytic activity of macrocyclic phenanthroline–Cu complexes. J Incl Phenom Macrocycl Chem 82, 437–451 (2015). https://doi.org/10.1007/s10847-015-0511-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-015-0511-1