Abstract
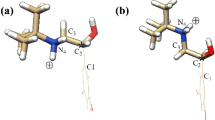
The diastereomeric complexation of both abacavir (ABA) and its enantiomer (ABAE) with (2-hydroxy)propyl-β-cyclodextrin (2HPβCD) with a degree of substitution of seven was studied. The apparent binding constants of diasteromeric complexes, ABA-2HPβCD and ABAE-2HPβCD were determined by ultra high-pressure liquid chromatography (UHPLC) and found to be 517.0 and 684.4 M−1 respectively. The stoichiometry of the complexes was determined by UHPLC and by the continuous variation method using nuclear magnetic resonance spectroscopy giving 1:1 complexes. The apparent binding constants decrease as the temperatures increases. The observed enantio-differentiation was analyzed theoretically by density functional theory at the PBE/6-31 g** level using a polarizable continuous model (PCM) for solvent effects, the most stable complexes are the ones in which the chiral cyclopentenyl moiety is included in the cavity of CD and the protonated purine ring interact with the hydroxypropyl groups of 2HPβCD. The differences in stability of diasteromeric complexes, due to different intermolecular interactions are consistent with experimental data, providing further insights in the formation of inclusion complexes.







Similar content being viewed by others
References
Global Industry Analysts, Inc.: Chiral technology: a global strategic business report. http://www.prweb.com/releases/chiral_technology/chiral_separation/prweb9369455.htm. Accessed 01 October 2014
Nguyen, L.A., He, H., Pham-Huy, C.: Chiral drugs: an overview. Int. J. Biomed. Sci. 2, 85–100 (2006)
Stalcup, A.M.: Chiral separations. Ann. Rev. Anal. Chem. 3, 341–363 (2010)
Cancelliere, G., Ciogli, A., D’Acquarica, I., Gasparrini, F., Kocergin, J., Misiti, D., Pierini, M., Ritchie, H., Simone, P., Villani, C.: Transition from enantioselective high performance to ultra-high performance liquid chromatography: a case study of a brush-type chiral stationary phase based on sub-5-micron to sub-2-micron silica particles. J. Chromatogr. A 1217, 990–999 (2010)
Hoffmann, C.V., Laemmerhofer, M., Lindner, W.: Novel strong cation-exchange type chiral stationary phase for the enantiomer separation of chiral amines by high-performance liquid chromatography. J. Chromatogr. A 1161, 242–251 (2007)
Ma, S., Shen, S., Haddad, N., Tang, W., Wang, J., Lee, H., Yee, N., Senanayake, C., Grinberg, N.: Chromatographic and spectroscopic studies on the chiral recognition of sulfated β-cyclodextrin as chiral mobile phase additive: enantiomeric separation of a chiral amine. J. Chromatogr. A 1216, 1232–1240 (2009)
Yu, L., Wang, S., Zeng, S.: Chiral mobile phase additives in HPLC enantioseparations. In: Scriba, G.K.E. (ed.) Chiral separations: methods and protocols, methods in molecular biology, vol. 970, pp. 221–231. Springer, Heidelberg (2013)
Nováková, L., Matysová, L., Solich, P.: Advantages of application of UPLC in pharmaceutical analysis. Talanta 68, 908–918 (2006)
Reyes-Reyes, M.L., Roa-Morales, G., Melgar-Fernández, R., Reyes-Pérez, H., Balderas-Hernández, P.: UHPLC determination of enantiomeric purity of sertraline in the presence of its production impurities. Chromatographia 77, 1315–1321 (2014)
Armstrong, D.W., Ward, T.J., Armstrong, R.D., Beesley, T.E.: Separation of drug stereoisomers by the formation of β-cyclodextrin inclusion complexes. Science 232, 1132–1135 (1986)
Lipkowitz, K.B., Coner, R., Peterson, M.A., Morreale, A., Shackelford, J.: The principle of maximum chiral discrimination: chiral recognition in permethyl-β-cyclodextrin. J. Org. Chem. 63, 732–745 (1998)
Foster, R.H., Faulds, D.: Abacavir. Drugs 55, 729–736 (1998)
Seshachalam, U., Narasimha-Rao, D.V.L., Haribabu, B., Chandrasekhar, K.B.: Chiral LC for separation of the enantiomers of abacavir sulfate. Chromatographia 64, 745–748 (2006)
Abacavir sulfate monograph in The United States Pharmacopoeial convention. USP 36 NF31. Baltimore, MD: United Book Press, Inc. (2013)
Grillo, R., Melo, N.F.S., Moraes, C.M., de Lima, R., Menezes, C.M.S., Ferreira, E.I., Rosa, A.H., Fernandes, L.F.: Study of the interaction between hydroxymethylnitrofurazone and 2-hydroxypropyl-β-cyclodextrin. J. Pharm. Biomed. Anal. 47, 295–302 (2008)
Fifere, A., Marangoci, N., Maier, S., Coroaba, A., Maftei, D., Pinteala, M.: Theoretical study on β-cyclodextrin inclusion complexes with propiconazole and protonated propiconazole. Beilstein J. Org. Chem. 8, 2191–2201 (2012)
Jana, M., Bandyopadhyay, S.: Molecular dynamics study of β-cyclodextrin–phenylalanine (1:1) inclusion complex in aqueous medium. J. Phys. Chem. B 117, 9280–9287 (2013)
Passos, J.J., De Sousa, F.B., Lula, I.S., Barreto, A.B., Lopes, J.F., De Almeida, W.B., Sinisterra, R.D.: Multi-equilibrium system based on sertraline and β-cyclodextrin supramolecular complex in aqueous solution. Int. J. Pharm. 42, 24–33 (2011)
Moraes, C.M., Abrami, P., de Paula, E., Braga, A.F.A., Fraceto, L.F.: Study of the interaction between S(−) bupivacaine and 2-hydroxypropyl-β-cyclodextrin. Int. J. Pharm. 331, 99 (2007)
Pîrnău, A., Mic, M., Bogdam, M., Turcu, I.: Characterization of β-cyclodextrin inclusion complex with procaine hydrochloride by 1H NMR and ITC. J. Incl. Phenom. Macrocycl. Chem. 79, 283–289 (2013)
Ufimtsev, I.S., Martinez, T.J.: Quantum chemistry on graphical processing units. 3. Analytical energy gradients and first principles molecular dynamics. J. Chem. Theory Comput. 5, 2619–2628 (2009)
Adamo, C., Barone, V.: Toward reliable density functional methods without adjustable parameters: the PBE0 method. J. Chem. Phys. 110, 6158–6170 (1999)
Gaussian 09, Revision D.01, Frisch, M. J., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., Scalmani, G., Barone, V., Mennucci, B., Petersson, G. A., Nakatsuji, H., Caricato, M., Li, X., Hratchian, H. P., Izmaylov, A. F., Bloino, J., Zheng, G., Sonnenberg, J. L., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Montgomery, J. A., Jr., Peralta, J. E., Ogliaro, F., Bearpark, M., Heyd, J. J., Brothers, E., Kudin, K. N., Staroverov, V. N., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A., Burant, J. C., Iyengar, S. S., Tomasi, J., Cossi, M., Rega, N., Millam, M. J., Klene, M., Knox, J. E., Cross, J. B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R. E., Yazyev, O., Austin, A. J., Cammi, R., Pomelli, C., Ochterski, J. W., Martin, R. L., Morokuma, K., Zakrzewski, V. G., Voth, G. A., Salvador, P., Dannenberg, J. J., Dapprich, S., Daniels, A. D., Farkas, Ö., Foresman, J. B., Ortiz, J. V., Cioslowski, J., Fox, D. J. Gaussian, Inc., Wallingford CT, (2009)
Scalmani, G., Frisch, J.M.: Continuous surface charge polarizable continuum models of solvation. I. General formalism. J. Chem. Phys. 132, 114110 (2010)
López-Nicolás, J.M.: García- Carmona, F.: Rapid, simple and sensitive determination of the apparent formation constants of trans-resveratrol complexes with natural cyclodextrins in aqueous medium using HPLC. Food Chem. 109, 868–875 (2008)
Filipa, M.A., Sancho, M.I., Gasull, E.I.: Determination of apparent binding constants by NSAIDs-βcyclodextrin complexes: HPLC, phase solubility diagrams and theoretical studies. J. Incl. Phenom. Macrocycl. Chem. 77, 223–230 (2013)
Parr, R.G., Yang, W.: Density functional theory of atoms and molecules. Oxford University Press, New York (1989)
Ireta, J., Neugebauer, J., Scheffler, M.: On the Accuracy of DFT for describing hydrogen bonds: dependence on the bond directionality. J. Phys. Chem. A 108, 5692–5698 (2004)
Arenzano, J.B., del Campo, J.M., Virues, J.O., Ramirez-Montes, P.I., Santillán, R., Rivera, J.M.: Theoretical study of the hydrogen bonding interaction between Levodopa and a new functionalized pillared coordination polymer designed as a carrier system. J. Mol. Struct. 1083, 106–110 (2015)
Yong, C.W., Washington, C., Smith, W.: Structural behavior of hydroxypropyl-β-cyclodextrin in water: molecular dynamic simulation studies. Pharm. Res. 25, 1092–1099 (2007)
Acknowledgments
Authors thanks to Secretaría de Investigación y Estudios Avanzados, Universidad Autónoma del Estado de México for the financial support trough the project 3864/2015PIC. Authors are also grateful to Signa S. A. de C. V., for some of the materials and instrumentation used during the development of this work.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Reyes-Reyes, M.L., Roa-Morales, G., Melgar-Fernández, R. et al. Chiral recognition of abacavir enantiomers by (2-hydroxy)propyl-β-cyclodextrin: UHPLC, NMR and DFT studies. J Incl Phenom Macrocycl Chem 82, 373–382 (2015). https://doi.org/10.1007/s10847-015-0499-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-015-0499-6