Abstract
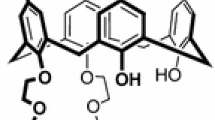
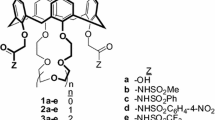
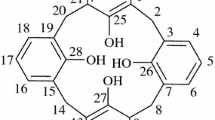
Di-ionizable calix[4]arene-1,3-crown-4 ligands with an enlarged crown ring were synthesized for comparison as metal ion extractants with analogues having identical pendent acidic groups, but a “normal” crown ring. The ligand conformation and regioselectivity are verified by NMR spectroscopy. Competitive solvent extraction of hard alkali metal cations and alkaline earth metal cations from aqueous solutions into chloroform by these new ligands were performed. Single species solvent extractions were conducted with intermediate Pb2+ and soft Hg2+. Comparison of the results with those from di-ionizable calix[4]arene-1,3-crown-4 ligands having “normal” crown rings provides insight into the effects of enlarging the crown ring as a function of the metal ion species being extracted and the identity of the pendent ionizable groups. Enlargement of the crown ring produced the largest increase in extraction efficiency with soft Hg2+.









Similar content being viewed by others
References
Gutsche, C.D.: Calixarenes Revisited, pp 89–90, 159–162. Royal Society of Chemistry, Cambridge (1998)
Gutsche, C.D. In: Lumetta, G.J., Rogers, R.D., Gopalan, A.S. (eds): Calixarenes for Separations: ACS Symposium vol. 757, pp 2–6. American Chemical Society, Washington, D.C. (2000)
Mandolini, L., Ungaro, R. (eds): Calixarenes in Action, pp 26, 64–65, 95–98, 146–152, 254–257. Imperial College Press, London (2000)
Casnati, A., Ungaro, R., Asfari, Z., Vincens, J, in Calixarenes 2001, Asfari, Z., Böhmer, V., Harrowfield, J., Vicens, J., pp 365–384. Kluwer Academic Publishers, Boston (2001)
Ungaro, R., Pochini, A.: New ionizable ligands from p-t-butylcalix[4]arene. J. Inclusion Phenom. 2, 199–206 (1984)
Zhou, H., Surowiec, K., Purkiss, D., Bartsch, R.A.: Proton di-ionizable p-tert-butylcalixarene-crown-6 compounds in cone and 1,3-alternate conformations: synthesis and alkaline earth cation extraction. Org. Biomol. Chem. 3, 1676–1684 (2005)
Zhou, H., Surowiec, K., Purkiss, D.W., Bartsch, R.A.: Synthesis and alkaline earth metal cation extraction by proton di-ionizable p-tert-butylcalix[4]arene-crown-5 compounds in cone, partial-cone, and 1,3-alternate conformations. Org. Biomol. Chem. 4, 1104–1114 (2006)
Tu, C., Surowiec, K., Bartsch, R.A.: Di-ionizable p-tert-butylcalix[4]arene-1,2-crown-4 ligands: synthesis and high divalent metal ion extraction selectivity. Tetrahedron Lett. 47, 3443–3446 (2006)
Tu, C., Liu, D., Surowiec, K., Purkiss, D.W., Bartsch, R.A.: Di-ionizable p-tert-butylcalix[4]arene-1,2-crown-5 and -crown-6 compounds in the cone conformation: synthesis and alkaline earth metal cation extraction. Org. Biomol. Chem. 4, 2938–2944 (2006)
Zhou, H., Liu, D., Gega, J., Surowiec, K., Purkiss, D.W., Bartsch, R.A.: Effect of para-substituents on alkaline earth metal ion extraction by proton di-ionizable calix[4]arene-crown-6 ligands in cone, partial-cone, and 1,3-alternate conformations. Org. Biomol. Chem. 5, 324–332 (2007)
Zhang, D., Cao, X., Purkiss, D.W., Bartsch, R.A., Zhang, D.: Di-ionizable p-tert-butylcalix[4]arene-1,2-crown-3 ligands in cone and 1,2-alternate conformations: Synthesis and metal ion extraction. Org. Biomol. Chem. 5, 1251–1259 (2007)
Tu, C., Surowiec, K., Bartsch, R.A.: Efficient divalent metal cation extractions with di-ionizable calix[4]-crown-4 compounds. Tetrahedron 63, 4184–4189 (2007)
Tu, C., Surowiec, K., Gega, J., Purkiss, D.W., Bartsch, R.A.: Di-ionizable calix[4]arene-1,2-crown-5 and -1,2-crown-6. Tetrahedron 64, 1187–1196 (2008)
Liu, X., Surowiec, K., Bartsch, R.A.: Di-ionizable p-tert-butylcalix[4]arene-1,3-crown-4 ligands: synthesis and alkaline earth metal cation extraction. Tetrahedron 65, 5893–5898 (2009)
Yang, Y., Arora, G., Fernandez, F.A., Crawford, J.D., Surowiec, K., Lee, E.K., Bartsch, R.A.: Lower-rim versus upper-rim functionalization in di-ionizable calix[4]arene-crown-5 isomers. Synthesis and divalent metal ion extraction. Tetrahedron 67, 1389–1397 (2011)
Yang, Y., Cao, X., Purkiss, D.W., Cannon, J.F., Bartsch, R.A.: Di-ionizable calix[4]areme-1,3-crown-4 ligands in 1,3-alternate, cone, and partial-cone conformations: synthesis and metal ion extractions. Tetrahedron 68, 2233–2244 (2012)
Boston, A.L., Lee, E.K., Crawford, J.D., Hanes Jr, R.E., Bartsch, R.A.: New di-ionizable p-tert-butylcalix[4]arene-1,2-crown-4 ligands. Synthesis and divalent metal ion extraction. Tetrahedron 68, 10241–10251 (2012)
Huber, V.J., Ivy, S.N., Lu, J., Bartsch, R.A.: Lariat ethers with a novel proton-ionizable group. Synthesis and solvent extraction of alkali metal cations. J. Chem. Soc., Chem. Commun. 1499–1500 (1997)
Casnati, A., Ca, N.D., Sansone, F., Ugozzoli, F., Ungaro, R.: Enlarging the size of calix[4]arene-crown-6 to improve Cs+/K+ selectivity: a theoretical and experimental study. Tetrahedron 60, 7869–7876 (2004)
Czech, B.P., Zazulak, W., Kumar, A., Dalley, N.K., Jiang, W., Bartsch, R.A.: Synthesis of benzo-13-crown-4 derivatives. J. Heterocycl. Chem. 28, 1387–1394 (1991)
Czech, B.P., Czech, A., Knudsen, B.E., Bartsch, R.A.: Synthesis and alkali metal cation by benzocrown ethers. Gazz. Chim. Ital. 117, 717–721 (1987)
Ouchi, M., Inoue, Y., Kanzaki, T., Hakushi, T.: Molecular design of crown ethers. 1. Effects of methylene chain length: 15- to 17-crown-5 and 18- to 22-crown-6. J. Org. Chem. 49, 1408–1412 (1984)
Gutsche, C.D.: Calixarenes, pp 106-115: Royal Society of Chemistry, Cambridge (1989)
Jaime, C., de Mendoza, J., Prados, P., Nieto, P.M.: Sanchez, C,: 13C NMR chemical shifts. A single rule to determine the conformation of calix[4]arene. J. Org. Chem. 56, 3372–3376 (1991)
Jolly, W.L.: Modern Inorganic Chemistry, p 208. McGraw-Hill, New York (1984)
Acknowledgments
We thank the Division of Chemical Sciences, Geosciences, and Biosciences of the Office of Basic Energy Sciences of the U.S. Department of Energy (Grant DE-FG02-9ER14416) for support of this research. We acknowledge NSF CRIF MU grant CHE-1048533 for an NMR spectrometer.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, Y., Lee, E.K. & Bartsch, R.A. Di-ionizable calix[4]arene-1,3-crown-4 ligands with an enlarged crown ring: synthesis and metal ion extraction. J Incl Phenom Macrocycl Chem 81, 367–376 (2015). https://doi.org/10.1007/s10847-014-0463-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-014-0463-x