Abstract
Purpose
Childbearing delay contributes to the increase of subfertile couples that require assisted reproductive technology (ART). Subfertility relates with reproductive aging (RA). In vitro aging (IvA) (due to extended culture) may also impair oocyte competence. Aims of this study were to evaluate and compare the oocyte ultrastructure after RA and IvA.
Methods
Cumulus-oocyte complexes (COCs) (n = 68), with metaphase II oocyte and expanded cumulus, from consenting patients (<35 years old and ≥35 years old, n = 36), were selected by phase contrast microscopy and fixed at pick up, or after 24 h culture. COCs (n = 44) were studied by light and qualitative/morphometric transmission electron microscopy. Two-way ANOVA, with age and culture as grouping factors, was applied for statistical analysis (p < 0.05). Metaphase II cumulus-free oocytes (n = 24) were selected for confocal microscopy observations.
Results
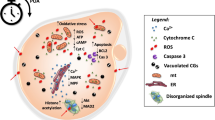
Significant decrease of mitochondria-smooth endoplasmic reticulum aggregates, increase of mitochondria-vesicle complexes size and amount, decrease of cortical granules and microvilli, and alterations of the spindle structure characterized both RA and IvA oocytes. These changes were significantly more evident in the RA oocytes submitted to IvA. RA oocytes also showed changes of the zona pellucida and occurrence of vacuoles after culture. Cumuli appeared re-compacted after culture, irrespective of the age of the patients.
Conclusions
These data demonstrated that aging is related to decay of oocyte ultrastructural quality, and that oocytes from elder women are more sensitive to prolonged culture (IvA) than the oocytes from younger women. These morphological results should be considered when applying ART in aged patients, rescue ICSI, or artificial oocyte activation.






Similar content being viewed by others
Abbreviations
- ART:
-
Assisted reproductive technology
- Ca++ :
-
Calcium
- COCs:
-
Cumulus-oocyte complexes
- CC cells:
-
Cumulus-corona cells
- CG:
-
Cortical granules
- E2:
-
Estradiol
- ICSI:
-
Intracytoplasmic sperm injection
- IVF:
-
In vitro fertilization
- IvA:
-
In vitro aging
- LM:
-
Light microscopy
- MII:
-
Metaphase II
- M-SER:
-
Mitochondria-smooth endoplasmic reticulum
- Mv:
-
Microvilli
- MV:
-
Mitochondria-vesicle
- PBI:
-
1st polar body
- PCM:
-
Phase contrast microscopy
- PVS:
-
Perivitelline space
- RA:
-
Reproductive aging
- s.c.:
-
Subcutaneous
- SER:
-
Smooth endoplasmic reticulum
- TEM:
-
Transmission electron microscopy
- US:
-
Ultrasound scan
- ZP:
-
Zona pellucida
- ZPt:
-
Zona pellucida thickness
References
Broekmans FJ, Soules MR, Fauser BC. Ovarian aging: mechanisms and clinical consequences. Endocr Rev. 2009;30:465–93.
Kocourkova J, Burcin B, Kucera T. Demographic relevancy of increased use of assisted reproduction in European countries. Reprod Health. 2014;26:11–37.
Miao YL, Kikuchi K, Sun QY, Schatten H. Oocyte aging: cellular and molecular changes, developmental potential and reversal possibility. Hum Reprod Update. 2009;15:573–85.
Leridon H. Can assisted reproduction technology compensate for the natural decline in fertility with age? A model assessment. Hum Reprod. 2004;19:1548–53.
Bentov Y, Yavorska T, Esfandiari N, Jurisicova A, Casper RF. The contribution of mitochondrial function to reproductive aging. J Assist Reprod Genet. 2011;28:773–83.
Qiao J, Wang ZB, Feng HL, Miao YL, Wang Q, Yu Y, et al. The root of reduced fertility in aged women and possible therapeutic options: current status and future prospects. Mol Aspects Med. 2013;38:54–85.
Navot D, Bergh PA, Williams MA, Garrisi GJ, Guzman I, Sandler B, et al. Poor oocyte quality rather than implantation failure as a cause of age-related decline in female fertility. Lancet. 1991;337:1375–7.
Battaglia DE, Goodwin P, Klein NA, Soules MR. Influence of maternal age on meiotic spindle assembly in oocytes from naturally cycling women. Hum Reprod. 1996;11:2217–22.
de Bruin JP, Dorland M, Spek ER, Posthuma G, van Haaften M, Looman CW, et al. Age-related changes in the ultrastructure of the resting follicle pool in human ovaries. Biol Reprod. 2004;70:419–24.
Wilcox AJ, Weinberg CR, Baird DD. Post-ovulatory aging of the human oocyte and embryo failure. Hum Reprod. 1998;13:394–7.
Chen C, Kattera S. Rescue ICSI of oocytes that failed to extrude the second polar body 6 h post-insemination in conventional IVF. Hum Reprod. 2003;18:2118–21.
Van Blerkom J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion. 2011;11:797–813.
Nasr-Esfahani MH, Deemeh MR, Tavalaee M. Artificial oocyte activation and intracytoplasmic sperm injection. Fertil Steril. 2010;94:520–6.
Beck-Fruchter R, Lavee M, Weiss A, Geslevich Y, Shalev E. Rescue intracytoplasmic sperm injection: a systematic review. Fertil Steril. 2014;101:690–8.
Coticchio G. Polarization microscopy and rescue ICSI. Reprod Biomed Online. 2013;26:222–3.
Stensen MH, Tanbo T, Storeng R, Byholm T, Fèdorcsak P. Routine morphological scoring systems in assisted reproduction treatment fail to reflect age-related impairment of oocyte and embryo quality. Reprod Biomed Online. 2010;21:118–25.
Khalili MA, Maione M, Palmerini MG, Bianchi S, Macchiarelli G, Nottola SA. Ultrastructure of human mature oocytes after vitrification. Eur J Histochem. 2012;56:e38.
Nottola SA, Macchiarelli G, Coticchio G, Bianchi S, Cecconi S, De Santis L, et al. Ultrastructure of human mature oocytes after slow cooling cryopreservation using different sucrose concentrations. Hum Reprod. 2007;22:1123–33.
Nottola SA, Coticchio G, De Santis L, Macchiarelli G, Maione M, Bianchi S, et al. Ultrastructure of human mature oocytes after slow cooling cryopreservation with ethylene glycol. Reprod Biomed Online. 2008;17:368–77.
Nottola SA, Coticchio G, Sciajno R, Gambardella A, Maione M, Scaravelli G, et al. Ultrastructural markers of quality in human mature oocytes vitrified using cryoleaf and cryoloop. Reprod Biomed Online. 2009;19:17–27.
Coticchio G, De Santis L, Rossi G, Borini A, Albertini D, Scaravelli G, et al. Sucrose concentration influences the rate of human oocytes with normal spindle and chromosome configurations after slow-cooling cryopreservation. Hum Reprod. 2006;21:1771–6.
Coticchio G, Borini A, Distratis V, Maione M, Scaravelli G, Bianchi V, et al. Qualitative and morphometric analysis of the ultrastructure of human oocytes cryopreserved by two alternative slow cooling protocols. J Assist Reprod Genet. 2010;27:131–40.
Shahedi A, Hosseini A, Khalili MA, Norouzian M, Salehi M, Piriaei A, et al. The effect of vitrification on ultrastructure of human in vitro matured germinal vesicle oocytes. Eur J Obstet Gynecol Reprod Biol. 2013;167:69–75.
Palmerini MG, Antinori M, Maione M, Cerusico F, Versaci C, Nottola SA, et al. Ultrastructure of immature and mature human oocytes after cryotop vitrification. J Reprod Dev. 2014;60:411–20.
Coticchio G, Sciajno R, Hutt K, Bromfield J, Borini A, Albertini DF. Comparative analysis of the metaphase II spindle of human oocytes through polarized light and high-performance confocal microscopy. Fertil Steril. 2010;93:2056–64.
Sundstrom P, Nilsson BO, Liedholm P, Larsson E. Ultrastructural characteristics of human oocytes fixed at follicular puncture or after culture. J In Vitro Fert Embryo Transf. 1985;2:195–206.
Sathananthan AH. Ultrastructure of the human egg. Hum Cell. 1997;10:21–38.
Volarcik K, Sheean L, Goldfarb J, Woods L, Abdul-Karim FW, Hunt P. The meiotic competence of in-vitro matured human oocytes is influenced by donor age: evidence that folliculogenesis is compromised in the reproductively aged ovary. Hum Reprod. 1998;13:154–60.
George MA, Pickering SJ, Braude PR, Johnson MH. The distribution of alpha- and gamma-tubulin in fresh and aged human and mouse oocytes exposed to cryoprotectant. Mol Hum Reprod. 1996;2:445–56.
Rienzi L, Balaban B, Ebner T, Mandelbaum J. The oocyte. Hum Reprod. 2012;27:i2–21.
Lin YH, Hwang JL, Huang LW, Seow KM, Hsieh BC, Tzeng CR. Comparison of Quinn’s Advantage fertilization medium and tissue culture medium 199 for in vitro maturation of oocytes. Taiwan J Obstet Gynecol. 2014;53:17–20.
Shih YF, Lee TH, Liu CH, Tsao HM, Huang CC, Lee MS. Effects of reactive oxygen species levels in prepared culture media on embryo development: a comparison of two media. Taiwan J Obstet Gynecol. 2014;53:504–8.
Motta PM, Nottola SA, Micara G, Familiari G. Ultrastructure of human unfertilized oocytes and polyspermic embryos in an IVF-ET program. Ann N Y Acad Sci. 1988;541:367–83.
Gualtieri R, Iaccarino M, Mollo V, Prisco M, Iaccarino S, Talevi R. Slow cooling of human oocytes: ultrastructural injuries and apoptotic status. Fertil Steril. 2009;91:1023–34.
El Shafie M, Sousa M, Windt M-L, Kruger TF. An atlas of the ultrastructure of human oocytes. New York, USA: Parthenon; 2000.
Bianchi V, Macchiarelli G, Borini A, Lappi M, Cecconi S, Miglietta S, et al. Fine morphological assessment of quality of human mature oocytes after slow freezing or vitrification with a closed device: a comparative analysis. Reprod Biol Endocrinol. 2014;12:110.
Sá R, Cunha M, Silva J, Luís A, Oliveira C, Teixeira da Silva J, et al. Ultrastructure of tubular smooth endoplasmic reticulum aggregates in human metaphase II oocytes and clinical implications. Fertil Steril. 2011;96:143–49.
Ebner T, Moser M, Shebl O, Sommerguber M, Tews G. Prognosis of oocytes showing aggregation of smooth endoplasmic reticulum. Reprod Biomed Online. 2008;16:113–8.
Otsuki J, Okada A, Morimoto K, Nagai Y, Kubo H. The relationship between pregnancy outcome and smooth endoplasmic reticulum clusters in MII human oocytes. Hum Reprod. 2004;19:1591–7.
Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26:1270–83.
Mateizel I, Van Landuyt L, Tournaye H, Verheyen G. Deliveries of normal healthy babies from embryos originating from oocytes showing the presence of smooth endoplasmic reticulum aggregates. Hum Reprod. 2013;28:2111–7.
Motta PM, Nottola SA, Makabe S, Heyn R. Mitochondrial morphology in human fetal and adult female germ cells. Hum Reprod. 2000;15:129–47.
Motta PM, Nottola SA, Familiari G, Makabe S, Stallone T, Macchiarelli G. Morphodynamics of the follicular-luteal complex during early ovarian development and reproductive life. Int Rev Cytol. 2003;223:177–288.
Van Blerkom J, Davis P, Mathwig V, Alexander S. Domains of high-polarized and low-polarized mitochondria may occur in mouse and human oocytes and early embryos. Hum Reprod. 2002;17:393–406.
Van Blerkom J, Davis P. Mitochondrial signaling and fertilization. Mol Hum Reprod. 2007;13:759–70.
Sousa M, Barros A, Silva J, Tesarik J. Developmental changes in calcium content of ultrastructurally distinct subcellular compartments of preimplantation human embryos. Mol Hum Reprod. 1997;3:83–90.
Swann K, Ozil JP. Dynamics of the calcium signal that triggers mammalian egg activation. Int Rev Cytol. 1994;152:183–222.
Nikiforaki D, Vanden Meerschaut F, Qian C, De Croo I, Lu Y, Deroo T, et al. Oocyte cryopreservation and in vitro culture affect calcium signalling during human fertilization. Hum Reprod. 2014;29:29–40.
Stricker SA. Structural reorganizations of the endoplasmic reticulum during egg maturation and fertilization. Semin Cell Dev Biol. 2006;17:303–13.
Ghadially FN. Ultrastructural pathology of the cell and matrix. 4th ed. Boston, USA: Butterworth-Heinemann; 1997.
McGinnis LK, Pelech S, Kinsey WH. Post-ovulatory aging of oocytes disrupts kinase signaling pathways and lysosome biogenesis. Mol Reprod Dev. 2014;81:928–45.
Muller-Hocker J, Schafer S, Weis S, Munscher C, Strowitzki T. Morphological-cytochemical and molecular genetic analysis of mitochondria in isolated human oocytes in the reproductive age. Mol Hum Reprod. 1996;2:951–8.
Van Blerkom J, Davis P, Lee J. ATP content of human oocytes and developmental potential and outcome after in-vitro fertilization and embryo transfer. Hum Reprod. 1995;10:415–24.
Sathananthan AH. Ultrastructure of human gametes, fertilization and embryos in assisted reproduction: a personal survey. Micron. 2013;44:1–20.
Familiari G, Heyn R, Relucenti M, Nottola SA, Sathananthan AH. Ultrastructural dynamics of human reproduction, from ovulation to fertilization and early embryo development. Int Rev Cytol. 2006;249:53–141.
Abbott AL, Xu Z, Kopf GS, Ducibella T, Schultz RM. In vitro culture retards spontaneous activation of cell cycle progression and cortical granule exocytosis that normally occur in in vivo unfertilized mouse eggs. Biol Reprod. 1998;59:1515–21.
Tarín JJ, Pérez-Albalá S, Cano A. Cellular and morphological traits of oocytes retrieved from aging mice after exogenous ovarian stimulation. Biol Reprod. 2001;65:141–50.
Díaz H, Esponda P. Aging-induced changes in the cortical granules of mouse eggs. Zygote. 2004;12:95–103.
Ducibella T, Duffy P, Reindollar R, Su B. Changes in the distribution of mouse oocyte cortical granules and ability to undergo the cortical reaction during gonadotropin-stimulated meiotic maturation and aging in vivo. Biol Reprod. 1990;43:870–6.
Sathananthan AH, Trounson AO. Ultrastructural observations on cortical granules in human follicular oocytes cultured in vitro. Gamete Res. 1982;5:191–8.
Ducibella T, Dubey A, Gross V, Emmi A, Penzias AS, Layman L, et al. A zona biochemical change and spontaneous cortical granule loss in eggs that fail to fertilize in in vitro fertilization. Fertil Steril. 1995;64:1154–61.
Talevi R, Gualtieri R, Tartaglione G, Fortunato A. Heterogeneity of the zona pellucida carbohydrate distribution in human oocytes failing to fertilize in vitro. Hum Reprod. 1997;12:2773–80.
Manna C, Rienzi L, Greco E, Sbracia M, Rahman A, Poverini R, et al. Zona pellucida solubility and cortical granule complements in human oocytes following assisted reproductive techniques. Zygote. 2001;9:201–10.
Familiari G, Nottola SA, Macchiarelli G, Micara G, Aragona C, Motta PM. Human zona pellucida during in vitro fertilization: an ultrastructural study using saponin, ruthenium red, and osmium-thiocarbohydrazide. Mol Reprod Dev. 1992;32:51–61.
Pickering SJ, Johnson MH, Braude PR, Houliston E. Cytoskeletal organization in fresh, aged and spontaneously activated human oocytes. Hum Reprod. 1988;3:978–89.
Kim NH, Chung HM, Cha KY, Chung KS. Microtubule and microfilament organization in maturing human oocytes. Hum Reprod. 1998;13:2217–22.
Coticchio G, Guglielmo MC, Albertini DF, Dal Canto M, Mignini Renzini M, De Ponti E, et al. Contributions of the actin cytoskeleton to the emergence of polarity during maturation in human oocytes. Mol Hum Reprod. 2014;20:200–7.
Sun QY, Schatten H. Regulation of dynamic events by microfilaments during oocyte maturation and fertilization. Reproduction. 2006;131:193–205.
Runge KE, Evans JE, He ZY, Gupta S, McDonald KL, Stahlberg H, et al. Oocyte CD9 is enriched on the microvillar membrane and required for normal microvillar shape and distribution. Dev Biol. 2007;304:317–25.
Sengoku K, Tamate K, Horikawa M, Takaoka Y, Ishikawa M, Dukelow WR. Plasma membrane block to polyspermy in human oocytes and preimplantation embryos. J Reprod Fertil. 1995;105:85–90.
Park KS, Song HB, Chun SS. Late fertilization of unfertilized human oocytes in in vitro fertilization and intracytoplasmic sperm injection cycles: conventional insemination versus ICSI. J Assist Reprod Genet. 2000;17:419–24.
Malter H, Talansky B, Gordon J, Cohen J. Monospermy and polyspermy after partial zona dissection of reinseminated human oocytes. Gamete Res. 1989;23:377–86.
Nottola SA, Makabe S, Stallone T, Familiari G, Correr S, Macchiarelli G. Surface morphology of the zona pellucida surrounding human blastocysts obtained after in vitro fertilization. Arch Histol Cytol. 2005;68:133–41.
Nawroth F, Muller P, Wolf C, Sudik R. Is the zona pellucida thickness of metaphase-II oocytes in an IVF/ICSI program influenced by the patient’s age? Gynecol Obstet Invest. 2001;52:55–8.
Kilani SS, Cooke S, Kan AK, Chapman MG. Do age and extended culture affect the architecture of the zona pellucida of human oocytes and embryos? Zygote. 2006;14:39–44.
Valeri C, Pappalardo S, De Felici M, Manna C. Correlation of oocyte morphometry parameters with woman’s age. J Assist Reprod Genet. 2011;28:545–52.
Nottola SA, Familiari G, Micara G, Aragona C, Motta PM. The ultrastructure of human cumulus-corona cells at the time of fertilization and early embryogenesis. A scanning and transmission electron microscopic study in an in vitro fertilization program. Arch Histol Cytol. 1991;54:145–61.
Motta PM, Nottola SA, Pereda J, Croxatto HB, Familiari G. Ultrastructure of human cumulus oophorus: a transmission electron microscopic study on oviductal oocytes and fertilized eggs. Hum Reprod. 1995;10:2361–7.
Motta PM, Nottola SA, Familiari G, Macchiarelli G, Correr S, Makabe S. Structure and function of the human oocyte-cumulus-corona cell complex before and after ovulation. Protoplasma. 1999;206:270–7.
McReynolds S, Dzieciatkowska M, McCallie BR, Mitchell SD, Stevens J, Hansen K, et al. Impact of maternal aging on the molecular signature of human cumulus cells. Fertil Steril. 2012;98:1574–80.
Bomsel-Helmreich O, Huyen LV, Durand-Gasselin I, Salat-Baroux J, Antoine JM. Mature and immature oocytes in large and medium follicles after clomiphene citrate and human menopausal gonadotropin stimulation without human chorionic gonadotropin. Fertil Steril. 1987;48:596–604.
Salhab M, Papillier P, Perreau C, Guyader-Joly C, Dupont J, Mermillod P, et al. Thymosins β-4 and β-10 are expressed in bovine ovarian follicles and upregulated in cumulus cells during meiotic maturation. Reprod Fertil Dev. 2010;22:1206–21.
Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet. 2001;2:280–91.
Eichenlaub-Ritter U, Stahl A, Luciani JM. The microtubular cytoskeleton and chromosomes of unfertilized human oocytes aged in vitro. Hum Genet. 1988;80:259–64.
Miyara F, Aubriot FX, Glissant A, Nathan C, Douard S, Stanovici A, et al. Multiparameter analysis of human oocytes at metaphase II stage after IVF failure in non-male infertility. Hum Reprod. 2003;18:1494–503.
Tsutsumi M, Fujiwara R, Nishizawa H, Ito M, Kogo H, Inagaki H, et al. Age-related decrease of meiotic cohesins in human oocytes. PLoS ONE. 2014;9:e96710.
Schatten H, Sun QY. Centrosome and microtubule functions and dysfunctions in meiosis: implications for age-related infertility and developmental disorders. Reprod Fertil Dev. 2015. doi:10.1071/RD14493.
Acknowledgments
The authors wish to acknowledge Prof. Stefano Necozione of the Department of Life, Health and Environmental Sciences, University of L’Aquila, for providing statistical advice and Mr. Ezio Battaglione of the Laboratory for Electron Microscopy “Pietro M. Motta”, Department of Anatomy, Histology, Forensic Medicine and Orthopaedics, “Sapienza” University, Rome, Italy, for his technical assistance.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Funding
The present study was supported by grants from the Italian Ministry of Education, University and Research (Sapienza and L’Aquila university grants), years 2011–2013.
Conflicts of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule
Aging is related to decay of oocyte ultrastructural quality. In vitro aging negatively affects morphofunctional features of mature oocytes in women of advanced age.
Rights and permissions
About this article
Cite this article
Bianchi, S., Macchiarelli, G., Micara, G. et al. Ultrastructural markers of quality are impaired in human metaphase II aged oocytes: a comparison between reproductive and in vitro aging. J Assist Reprod Genet 32, 1343–1358 (2015). https://doi.org/10.1007/s10815-015-0552-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-015-0552-9