Abstract
Purpose
Perform the genetic characterization of five patients with total sperm immotility using Sanger sequencing and Whole Exome Sequencing (WES), in order to increase the knowledge on the genetics of sperm immotility and, ultimately, allow the identification of potential genetic markers for infertility.
Methods
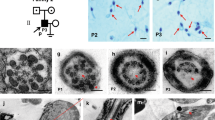
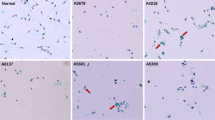
Prospective study at a University Medical school. We analysed five men with total sperm immotility, four with dysplasia of the fibrous sheath (DFS), associated with disruption of several axonemal structures, and one patient with situs inversus totalis, which showed absence of dynein arms (DA) and nexin bridges. We screened 7 genes by Sanger sequencing, involved in sperm motility and associated to ultrastructural defects found in these patients (CCDC39, CCDC40, DNAH5, DNAI1, RSPH1, AKAP3 and AKAP4). Additionally, we performed WES analysis in the patient with situs inversus.
Results
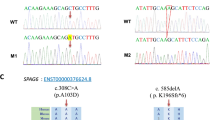
We identified nine new DNA sequence variants by WES. Two of these variants were considered particularly relevant: a homozygous missense change in CCDC103 gene (c.104G > C, p.R35P) probably related with absence of dynein arms; the other in the INSL6 gene (c.262_263delCC) is thought to be also involved in sperm immotility.
Conclusions
Our work suggests that WES is an effective strategy, especially as compared with conventional sequencing, to study highly heterogenic genetic diseases, such as sperm immotility. For future work we expect to expand the analysis of WES to the other four patients and complement findings with expression analysis or functional studies to determine the impact of the novel variants.

Similar content being viewed by others
References
Curry MR, Watson PF. Sperm structure and function. In: Grudzinskas JG, Yovich JL, editors. Gametes - the spermatozoon. NY: Cambridge University Press; 1995. p. 45–69.
Eddy EM, Toshimori K, O’Brien DA. Fibrous sheath of mammalian spermatozoa. Microsc Res Tech. 2003;61:103–15.
Burgess SA, Knight PJ. Is the dynein motor a winch? Curr Opin Struct Biol. 2004;14:138–46.
Smith EF, Yang P. The radial spokes and central apparatus: mechano-chemical transducers that regulate flagellar motility. Cell Motil Cytoskeleton. 2004;57:8–17.
Wirschell M, Olbrich H, Werner C, Tritschler D, Bower R, Sale W, et al. The nexin-dynein regulatory complex subunit DRC1 is essential for motile cilia function in algae and humans. Nat Genet. 2013;45:262–8.
Ortega C, Verheyen G, Raick D, Camus M, Devroey P, Tournaye H. Absolute asthenozoospermia and ICSI: what are the options? Hum Reprod Update. 2011;17(5):684–92.
Afzelius BA. The immotile-cilia syndrome: a microtubule-associated defect. Crit Rev Biochem Mol Biol. 1985;19:63–87.
Boon M, Jorissen M, Proesmans M, De Boeck K. Primary ciliary dyskinesia, an orphan disease. Eur J Pediatr. 2013;172:151–62.
Leigh MW, Pittman JE, Carson JL, Ferkol TW, Dell S, Davis SD, et al. Clinical and genetic aspects of primary ciliary dyskinesia/Kartagener syndrome. Genet Med. 2009;11:473–87.
Pereira R, Oliveira J, Sousa M. A molecular approach to sperm immotility in humans: a review. Med Reprod y Embriol Clín. 2014;01(01):15–25.
Djakow J, Svobodová T, Hrach K, Uhlík J, Cinek O, Pohunek P. Effectiveness of sequencing selected exons of DNAH5 and DNAI1 in diagnosis of primary ciliary dyskinesia. Pediatr Pulmonol. 2012;47:864–75.
Merveille A-C, Davis EE, Becker-Heck A, Legendre M, Amirav I, Bataille G, et al. CCDC39 is required for assembly of inner dynein arms and the dynein regulatory complex and for normal ciliary motility in humans and dogs. Nat Genet. 2011;43:72–8.
Becker-Heck A, Zohn IE, Okabe N, Pollock A, Lenhart KB, Sullivan-Brown J, et al. The coiled-coil domain containing protein CCDC40 is essential for motile cilia function and left-right axis formation. Nat Genet. 2011;43:79–84.
Antony D, Becker-Heck A, Zariwala MA, Schmidts M, Onoufriadis A, Forouhan M, et al. Mutations in CCDC39 and CCDC40 are the major cause of primary ciliary dyskinesia with axonemal disorganization and absent inner dynein arms. Hum Mutat. 2013;34:462–72.
Kott E, Legendre M, Copin B, Papon J-F, Dastot-Le Moal F, Montantin G, et al. Loss-of-function mutations in rsph1cause primary ciliary dyskinesia with central-complex and radial-spoke defects. Am J Hum Genet. 2013;93:561–70.
Chemes HE, Olmedo SB, Carrere C, Oses R, Carizza C, Leisner M, et al. Ultrastructural pathology of the sperm flagellum: association between flagellar pathology and fertility prognosis in severely asthenozoospermic men. Hum Reprod. 1998;13:2521–6.
Chemes HE, Rawe VY. The making of abnormal spermatozoa: cellular and molecular mechanisms underlying pathological spermiogenesis. Cell Tissue Res. 2010;341:349–57.
Luconi M, Cantini G, Baldi E, Forti G. Role of a-kinase anchoring proteins (AKAPs) in reproduction. Front Biosci. 2011;16:1315–30.
Turner RMO, Johnson LR, Haig-Ladewig L, Gerton GL, Moss SB. An X-linked gene encodes a major human sperm fibrous sheath protein, hAKAP82: genomic organization, protein kinase a-rii binding, and distribution of the precursor in the sperm tail. J Biol Chem. 1998;273:32135–41.
Mandal A, Naaby-Hansen S, Wolkowicz MJ, Klotz K, Shetty J, Retief JD, et al. FSP95, a testis-specific 95-kilodalton fibrous sheath antigen that undergoes tyrosine phosphorylation in capacitated human spermatozoa. Biol Reprod. 1999;61:1184–97.
Baccetti B, Collodel G, Gambera L, Moretti E, Serafini F, Piomboni P. Fluorescence in situ hybridization and molecular studies in infertile men with dysplasia of the fibrous sheath. Fertil Steril. 2005;84:123–9.
Baccetti B, Collodel G, Estenoz M, Manca D, Moretti E, Piomboni P. Gene deletions in an infertile man with sperm fibrous sheath dysplasia. Hum Reprod. 2005;20:2790–4.
Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215.
Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden TL. Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics. 2012;13:134.
Gaspar P, Lopes P, Oliveira J, Santos R, Dalgleish R, Oliveira JL. Variobox: automatic detection and annotation of human genetic variants. Hum Mutat. 2014;35(2):202–7.
Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–9.
Ng PC, Henikoff S. SIFT: predicting amino acid changes that affect protein function. Nucleic Acids Res. 2003;31:3812–4.
Schwarz JM, Rödelsperger C, Schuelke M, Seelow D. MutationTaster evaluates disease-causing potential of sequence alterations. Nat Methods. 2010;7:575–6.
Desmet F-O, Hamroun D, Lalande M, Collod-Béroud G, Claustres M, Béroud C. Human splicing finder: an online bioinformatics tool to predict splicing signals. Nucleic Acids Res. 2009;37:e67.
Oliveira J, Negrao L, Fineza I, Taipa R, Melo-Pires M, Fortuna AM, et al. New splicing mutation in the choline kinase beta (CHKB) gene causing a muscular dystrophy detected by whole-exome sequencing. J Hum Genet. 2015. doi:10.1038/jhg.2015.20.
Paila U, Chapman BA, Kirchner R, Quinlan AR. GEMINI: integrative exploration of genetic variation and genome annotations. PLoS Comput Biol. 2013;9, e1003153.
Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, et al. Gene ontology: tool for the unification of biology. Nat Genet. 2000;25:25–9.
Ewing B, Hillier L, Wendl MC, Green P. Base-calling of automated sequencer traces usingPhred. I. Accuracy assessment. Genome Res. 1998;8:175–85.
Ewing B, Green P. Base-calling of automated sequencer traces using phred. II. Error probabilities. Genome Res. 1998;8:186–94.
Doggett NA, Xie G, Meincke LJ, Sutherland RD, Mundt MO, Berbari NS, et al. A 360-kb interchromosomal duplication of the human HYDIN locus. Genomics. 2006;88:762–71.
Berg JS, Evans JP, Leigh MW, Omran H, Bizon C, Mane K, et al. Next generation massively parallel sequencing of targeted exomes to identify genetic mutations in primary ciliary dyskinesia: implications for application to clinical testing. Genet Med. 2011;13:218–29.
Franceschini A, Szklarczyk D, Frankild S, Kuhn M, Simonovic M, Roth A, et al. STRING v9. 1: protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 2013;41:D808–15.
Hornef N, Olbrich H, Horvath J, Zariwala MA, Fliegauf M, Loges NT, et al. DNAH5 mutations are a common cause of primary ciliary dyskinesia with outer dynein arm defects. Am J Respir Crit Care Med. 2006;174:120–6.
Schwabe GC, Hoffmann K, Loges NT, Birker D, Rossier C, De Santi MM, et al. Primary ciliary dyskinesia associated with normal axoneme ultrastructure is caused by DNAH11 mutations. Hum Mutat. 2008;29:289–98.
Knowles MR, Leigh MW, Carson JL, Davis SD, Dell SD, Ferkol TW, et al. Mutations of DNAH11 in patients with primary ciliary dyskinesia with normal ciliary ultrastructure. Thorax. 2012;67:433–41.
Miki K, Willis WD, Brown PR, Goulding EH, Fulcher KD, Eddy EM. Targeted disruption of the Akap4 gene causes defects in sperm flagellum and motility. Dev Biol. 2002;248:331–42.
Turner RMO, Musse MP, Mandal A, Klotz KEN, Friederike C, Jayes L, et al. Molecular genetic analysis of two human sperm fibrous. J Androl. 2001;22:302–15.
Moretti E, Scapigliati G, Pascarelli NA, Baccetti B, Collodel G. Localization of AKAP4 and tubulin proteins in sperm with reduced motility. Asian J Androl. 2007;9:641–9.
Colantonio JR, Vermot J, Wu D, Langenbacher AD, Fraser S, Chen J-N, et al. The dynein regulatory complex is required for ciliary motility and otolith biogenesis in the inner ear. Nature. 2008;457:205–9.
Yeh S-D, Chen Y-J, Chang ACY, Ray R, She B-R, Lee W-S, et al. Isolation and properties of Gas8, a growth arrest-specific gene regulated during male gametogenesis to produce a protein associated with the sperm motility apparatus. J Biol Chem. 2002;277:6311–7.
Bekker JM, Colantonio JR, Stephens AD, Clarke WT, King SJ, Hill KL, et al. Direct interaction of Gas11 with microtubules: implications for the dynein regulatory complex. Cell Motil Cytoskeleton. 2007;64:461–73.
Asai DJ, Koonce MP. The dynein heavy chain: structure, mechanics and evolution. Trends Cell Biol. 2001;11:196–202.
Silvanovich A, Li M, Serr M, Mische S, Hays TS. The third P-loop domain in cytoplasmic dynein heavy chain is essential for dynein motor function and ATP-sensitive microtubule binding. Mol Biol Cell. 2003;14:1355–65.
Zukas R, Chang AJ, Rice M, Springer AL. Structural analysis of flagellar axonemes from inner arm dynein knockdown strains of Trypanosoma brucei. Biocell. 2012;36:133–42.
Panizzi JR, Becker-heck A, Castleman VH, Al-mutairi D, Liu Y, Loges NT, et al. CCDC103 mutations cause primary ciliary dyskinesia by disrupting assembly of ciliary dynein arms. Nat Genet. 2012;44:714–9.
Anand-Ivell R, Dai Y, Ivell R. Neohormones as biomarkers of reproductive health. Fertil Steril. 2013;99:1153–60.
Lok S, Johnston DS, Conklin D, Lofton-Day CE, Adams RL, Jelmberg AC, et al. Identification of INSL6, a new member of the insulin family that is expressed in the testis of the human and rat. Biol Reprod. 2000;62:1593–9.
Burnicka-Turek O, Shirneshan K, Paprotta I, Grzmil P, Meinhardt A, Engel W, et al. Inactivation of insulin-like factor 6 disrupts the progression of spermatogenesis at late meiotic prophase. Endocrinology. 2009;150:4348–57.
Chen G-W, Luo X, Liu Y-L, Jiang Q, Qian X-M, Guo Z-Y. R171H missense mutation of INSL6 in a patient with spermatogenic failure. Eur J Med Genet. 2011;54:e455–7.
Knowles MR, Leigh MW, Ostrowski LE, Huang L, Carson JL, Hazucha MJ, et al. Exome sequencing identifies mutations in CCDC114as a cause of primary ciliary dyskinesia. Am J Hum Genet. 2013;92:99–106.
Onoufriadis A, Shoemark A, Munye MM, James CT, Schmidts M, Patel M, et al. Combined exome and whole-genome sequencing identifies mutations in ARMC4 as a cause of primary ciliary dyskinesia with defects in the outer dynein arm. J Med Genet BMJ Publishing Group Ltd; 2014;51:61–7.
Bamshad MJ, Ng SB, Bigham AW, Tabor HK, Emond MJ, Nickerson DA, et al. Exome sequencing as a tool for Mendelian disease gene discovery. Nat Rev Genet. 2011;12:745–55.
Teves ME, Zhang Z, Costanzo RM, Henderson SC, Corwin FD, Zweit J, et al. Sperm-associated antigen-17 gene is essential for motile cilia function and neonatal survival. Am J Respir Cell Mol Biol. 2013;48:765–72.
Acknowledgements
We would like to acknowledge: Helena Oliveira, MSc, ESHRE Senior clinical embryologist; Ilda Pires, MSc, ESHRE Senior clinical embryologist and Madalena Cabral, BSc, Embryologist, for semen processing at CHVNG; Ana Gonçalves, BSc, and Cláudia Osório, BSc, for semen processing at CGR; Elsa Oliveira, 1st Class Technical Specialist of Pathology, Cytology and Thanatology in the Area of Diagnosis and Therapy and Ângela Alves, Technical assistant teaching and research, for semen processing for electron microscopy at ICBAS-UP.
We also would like to acknowledge Conceição Egas, PhD and Hugo Froufe, MSc (GenoInseq) for performing exome sequencing and assisting the initial variant filtering/analysis.
Funding
This work was financed by the Institutions of the authors and in part by UMIB, which is funded by National Funds through FCT-Foundation for Science and Technology, under the Pest-OE/SAU/UI0215/2014.
Disclosure statement
The authors have nothing to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule We studied four patients with fibrous sheath dysplasia and one with situs inversus totalis, all with total sperm immotility. Nine new DNA sequence variants were identified. Whole Exome Sequencing (WES) revealed to be the most efficient procedure for the genetic analysis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Data 1
(DOCX 30 kb)
Supplementary Figure 1
(DOCX 36 kb)
Supplementary Figure 2
(DOCX 147 kb)
Rights and permissions
About this article
Cite this article
Pereira, R., Oliveira, J., Ferraz, L. et al. Mutation analysis in patients with total sperm immotility. J Assist Reprod Genet 32, 893–902 (2015). https://doi.org/10.1007/s10815-015-0474-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-015-0474-6