Abstract
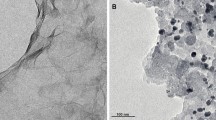
A series of platinum nanoparticle-loaded carbon composites composed of reduced graphene oxide and multi-walled carbon nanotubes (rGO-CNT/Pt) was chemically synthesized at room temperature. The prepared catalysts were characterized using X-ray diffraction (XRD), Raman spectroscopy, scanning electron microscopy (SEM), transmission electron microscopy (TEM) and X-ray photoelectron spectroscopy (XPS). The results confirm that rGO mixed CNT composites were successfully prepared with small and highly dispersed Pt nanoparticles on the composite supports. The cyclic voltammetry (CV) and chronoamperometry (CA) electrochemical measurements indicated that the composite carbon catalyst increased the intensity and stability for the methanol oxidation reaction (MOR). In addition, fewer carbon intermediate species were observed in the CO stripping experiment. The incorporation of CNTs into rGO decreases the agglomeration between rGO sheets and increases the active surface of dispersed Pt nanoparticles, resulting in the enhanced oxidation activity of the as-prepared electrocatalysts. The combined characteristics of the highly active Pt nanoparticles and potent electron transfer of the rGO-CNTs enable excellent methanol oxidation with high stability. Consequently, this hybrid carbon material is a good candidate for MOR catalysis, which can be applied to direct methanol fuel cells.
Graphic abstract








Similar content being viewed by others
References
Kamarudin SK, Achmad F, Daud WRW (2009) Overview on the application of direct methanol fuel cell (DMFC) for portable electronic devices. Int J Hydrog Energy 34:6902–6916. https://doi.org/10.1016/j.ijhydene.2009.06.013
Lamy C, Coutanceau C (2012) Chapter 1 electrocatalysis of alcohol oxidation reactions at platinum group metals. In: Catalysts for alcohol-fuelled direct oxidation fuel cells. The Royal Society of Chemistry, pp 1–70. https://doi.org/10.1007/s11244-018-0893-6
Ramli ZAC, Kamarudin SK (2018) Platinum-based catalysts on various Carbon supports and conducting polymers for direct methanol fuel cellapplications: a review. Nanoscale Res Lett 13:410. https://doi.org/10.1186/s11671-018-2799-4
Pereira JP, Falcão DS, Oliveira VB, Pinto AMFR (2014) Performance of a passive direct ethanol fuel cell. J Power Sources 256:14–19. https://doi.org/10.1016/j.jpowsour.2013.12.036
Zhou W (2003) Pt based anode catalysts for direct ethanol fuel cells. Appl Catal B Environ 46:273–285. https://doi.org/10.1016/S0926-3373(03)00218-2
Liu M, Zhang R, Chen W (2014) Graphene-supported nanoelectrocatalysts for fuel cells: synthesis, properties, and applications. Chem Rev 114:5117–5160. https://doi.org/10.1021/cr400523y
Samad S, Loh KS, Wong WY et al (2018) Carbon and non-carbon support materials for platinum-based catalysts in fuel cells. Int J Hydrog Energy 43:7823–7854. https://doi.org/10.1016/j.ijhydene.2018.02.154
Chung DY, Lee KJ, Sung YE (2016) Methanol electro-oxidation on the Pt surface: revisiting the cyclic voltammetry interpretation. J Phys Chem C. https://doi.org/10.1021/acs.jpcc.5b12303
Parkinson CR, Walker M, McConville CF (2003) Reaction of atomic oxygen with a Pt(1 1 1) surface: chemical and structural determination using XPS, CAICISS and LEED. Surf Sci 545:19–33. https://doi.org/10.1016/j.susc.2003.08.029
Sakong S, Groß A (2017) Methanol oxidation on Pt(111) from first-principles in heterogeneous and electrocatalysis. Electrocatalysis 8:577–586. https://doi.org/10.1007/s12678-017-0370-1
Li M, Liu P, Adzic RR (2012) Platinum monolayer electrocatalysts for anodic oxidation of alcohols. J Phys Chem Lett 3:3480–3485. https://doi.org/10.1021/jz3016155
Ozkaya D (2008) Particle size analysis of supported platinum catalysts by TEM. Platin Met Rev 52:61–62. https://doi.org/10.1595/147106708X264095
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans (2017) Carbon nanotubes; Iarc Monographs—111. 1991. https://www.ncbi.nlm.nih.gov/books/NBK436610/
Twigg MV (2009) Carbons and carbon supported catalysts in hydroprocessing. Platin Met Rev 53:135–137. https://doi.org/10.1595/147106709X465479
Khan M, Tahir MN, Adil SF et al (2015) Graphene based metal and metal oxide nanocomposites: synthesis, properties and their applications. J Mater Chem A 3:18753–18808. https://doi.org/10.1039/c5ta02240a
Sudirman S, Indriyati I, Adi WA et al (2017) Structural analysis of platinum nanoparticles on carbon nanotube surface as electrocatalyst system. Int J Chem 9:60. https://doi.org/10.5539/ijc.v9n2p60
Ramli ZAC, Kamarudin SK (2018) Platinum-based catalysts on various carbon supports and conducting polymers for direct methanol fuel cell applications: a review. Nanoscale Res Lett. https://doi.org/10.1186/s11671-018-2799-4
Jha N, Jafri RI, Rajalakshmi N, Ramaprabhu S (2011) Graphene-multi walled carbon nanotube hybrid electrocatalyst support material for direct methanol fuel cell. Int J Hydrog Energy 36:7284–7290. https://doi.org/10.1016/j.ijhydene.2011.03.008
Fan W, Zhang L, Liu T (2017) Graphene-carbon nanotube hybrids for energy and environmental applications. Springer, Singapore, pp 21–51. https://doi.org/10.1007/978-981-10-2803-8
Kwok YH, Wang YF, Tsang ACH, Leung DYC (2018) Graphene-carbon nanotube composite aerogel with Ru@Pt nanoparticle as a porous electrode for direct methanol microfluidic fuel cell. Appl Energy 217:258–265. https://doi.org/10.1016/j.apenergy.2018.02.141
Pham K-C, McPhail DS, Mattevi C et al (2016) Graphene-carbon nanotube hybrids as robust catalyst supports in proton exchange membrane fuel cells. J Electrochem Soc 163:F255–F263. https://doi.org/10.1149/2.0891603jes
Zhang X, Zhang J, Huang H et al (2017) Platinum nanoparticles anchored on graphene oxide-dispersed pristine carbon nanotube supports: high-performance electrocatalysts toward methanol electrooxidation. Electrochim Acta 258:919–926. https://doi.org/10.1016/j.electacta.2017.11.142
Wise AM, Richardson PW, Price SWT et al (2018) Inhibitive effect of Pt on Pd-hydride formation of Pd@Pt core-shell electrocatalysts: an in situ EXAFS and XRD study. Electrochim Acta 262:27–38. https://doi.org/10.1016/j.electacta.2017.12.161
Edelstein AS (2001) Nanomaterials. Encycl Mater Sci Technol. https://doi.org/10.1016/B0-08-043152-6/01031-7
Bin WuJ, Lin ML, Cong X et al (2018) Raman spectroscopy of graphene-based materials and its applications in related devices. Chem Soc Rev 47:1822–1873. https://doi.org/10.1039/c6cs00915h
Wang X, Wang H, Wang R et al (2012) Carbon-supported platinum-decorated nickel nanoparticles for enhanced methanol oxidation in acid media. J Solid State Electrochem 16:1049–1054. https://doi.org/10.1007/s10008-011-1485-6
Fujimoto A, Yamada Y, Koinuma M, Sato S (2016) Origins of sp 3 C peaks in C 1s X-ray photoelectron spectra of carbon materials. Anal Chem 88:6110–6114. https://doi.org/10.1021/acs.analchem.6b01327
Themsirimongkon S, Ounnunkad K, Saipanya S (2018) Electrocatalytic enhancement of platinum and palladium metal on polydopamine reduced graphene oxide support for alcohol oxidation. J Colloid Interface Sci 530:98–112. https://doi.org/10.1016/j.jcis.2018.06.072
Jonathan JB, Steven KB (2013) Electrodeposition of Pt nanoparticle catalysts from H2Pt(OH)6 and their application in PEM Fuel Cells. J Phys Chem C 117:18957–18966. https://doi.org/10.1021/jp405302x
Iwasita T (2010) Methanol and CO electrooxidation. Handb Fuel Cells 2:603–624. https://doi.org/10.1002/9780470974001.f206047
Liu G, Pan Z, Li W et al (2017) The effect of titanium nickel nitride decorated carbon nanotubes-reduced graphene oxide hybrid support for methanol oxidation. Appl Surf Sci 410:70–78. https://doi.org/10.1016/j.apsusc.2017.03.075
Eichhorn B, Tsypkin M, Seland F et al (2011) CO stripping as an electrochemical tool for characterization of Ru@Pt core-shell catalysts. J Electroanal Chem 655:140–146. https://doi.org/10.1016/j.jelechem.2011.02.027
Calderón JC, García G, Calvillo L et al (2015) Electrochemical oxidation of CO and methanol on Pt-Ru catalysts supported on carbon nanofibers: the influence of synthesis method. Appl Catal B Environ 165:676–686. https://doi.org/10.1016/j.apcatb.2014.10.077
Mu X, Xu Z, Ma Y, Xie Y, Mi H, Ma J (2017) Graphene-carbon nanofiber hybrid supported Pt nanoparticles with enhanced catalytic performance for methanol oxidation and oxygen reduction. Electrochim Acta 253:171–177. https://doi.org/10.1016/j.electacta.2017.09.029
Gorle DB, Kulandainathan MA (2017) One-pot synthesis of highly efficient graphene based three-dimensional hybrids as catalyst supporting materials for electro-oxidation of liquid fuels. J Mater Chem A 5:15273–15286. https://doi.org/10.1039/C7TA03276E
Zhao L, Wang Z-B, Li J-L, Zhang J-J, Sui X-L, Zhang L-M (2016) Hybrid of carbon-supported Pt nanoparticles and three-dimensional graphene aerogel as high stable electrocatalyst for methanol electrooxidation. Electrochim Acta 189:175–183. https://doi.org/10.1016/j.electacta.2015.12.072
Yan H, Meng M, Wang L, Wu A, Tian C, Zhao L, Fu H (2016) Small-sized tungsten nitride anchoring into a 3D CNTrGO framework as a superior bifunctional catalyst for the methanol oxidation and oxygen reduction reactions. Nano Res 9:329–343. https://doi.org/10.1007/s12274-015-0912-x
Yi G, Chang Z, Liu G, Yang L (2019) In situ fabrication of copper nanocubes with platinum skin on 3D graphene-carbon nanotubes hybrid for efficient methanol electrooxidation. Int J Electrochem Sci 14:7232–7240. https://doi.org/10.20964/2019.08.25
Yan M, Jiang Q, Zhang T, Wang J, Yang L, Lu Z, He H, Fu Y, Wang X, Huang H (2018) Three-dimensional low-defect carbon nanotube/nitrogen-doped graphene hybrid aerogel-supported Pt nanoparticles as efficient electrocatalysts toward the methanol oxidation reaction. J Mater Chem A 6:18165–18172. https://doi.org/10.1039/c8ta05124k
Mani V, Chen SM, Lou BS (2013) Three dimensional graphene oxide-carbon nanotubes and graphene-carbon nanotubes hybrids. Int J Electrochem Sci 8:11641–11660
Acknowledgements
This work was supported by Centre of Excellence in Materials Science and Technology (CoE), Environmental Science Research Centre (ESRC), CMU Mid-Career Research Fellowship program, Graduate School, Faculty of Science, Chiang Mai University, and Science Achievement Scholarship of Thailand (SAST) for financial and experimental set-up.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pongpichayakul, N., Waenkeaw, P., Jakmunee, J. et al. Activity and stability improvement of platinum loaded on reduced graphene oxide and carbon nanotube composites for methanol oxidation. J Appl Electrochem 50, 51–62 (2020). https://doi.org/10.1007/s10800-019-01368-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-019-01368-1