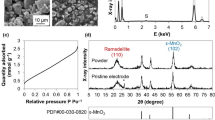
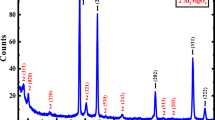
Abstract
The impact of chemical additives [e.g., BaSO4, Sr(OH)2·8H2O, Ca(OH)2, and Bi2O3] on the cycling performance of rechargeable alkaline electrolytic manganese dioxide/Zn batteries has been studied. The additives were used in the cathode electrodes consisting of 5 wt% additive, γ-MnO2 (electrolytic manganese dioxide—EMD—80 wt%) and KS44 graphite (15 wt%) in prismatic-type electrode geometries. Powder X-ray diffraction analysis showed the formation of alkaline earth metal carbonates (BaCO3, SrCO3) when the electrodes come into contact with the highly alkaline (pH 15, 9 M KOH) electrolyte. The presence of dissolved carbonate in the electrolyte leads to a double replacement precipitation reaction leading to the formation of these carbonate species. These additives help with the cycle life of the electrolytic manganese dioxide cathode material. The overall energy efficiency of the cells is about 75%. Rate capability studies and equilibrium potential measurements by galvanostatic intermittent titration technique analysis indicate that ohmic polarization plays a significant role in the energy loss and should be improved for high power applications. Rechargeable alkaline batteries with electrolytic manganese dioxide/Zn chemistry provide a low-cost and an environmentally friendly solution for storage of energy. Improvement of this technology would be an important contribution in the area of energy storage applications.
Graphical Abstract
The impact of a number of chemical additives (e.g., BaSO4, Sr(OH)2·8H2O, Ca(OH)2 and Bi2O3) on the cycling performance of rechargeable alkaline EMD/Zn batteries has been studied. The additives were used in the cathode electrodes consisting of 5 wt% additive, γ-MnO2 (electrolytic manganese dioxide—EMD—80 wt%) and KS44 graphite (15 wt%) in prismatic-type electrode geometries. Powder X-ray diffraction analysis showed the formation of alkaline earth metal carbonates (BaCO3, SrCO3) when the electrodes come into contact with the highly alkaline (pH 15, 9 M KOH) electrolyte. The presence of dissolved carbonate in the electrolyte leads to a double replacement precipitation reaction leading to the formation of these carbonate species. These additives help with the cycle life of the EMD cathode material. The overall energy efficiency of the cells is about 75%. Rate capability studies and equilibrium potential measurements by galvanostatic intermittent titration technique analysis indicate that ohmic polarization plays a significant role in the energy loss and should be improved for high power applications. Rechargeable alkaline batteries with EMD/Zn chemistry provide a low-cost and an environmentally friendly solution for storage of energy. Improvement of this technology would be an important contribution in the area of energy storage applications.














Similar content being viewed by others
References
Larcher D, Tarascon J-M (2014) Towards greener and more sustainable batteries for electrical energy storage. Nat Chem 7:19–29. doi:10.1038/nchem.2085
Dunn B, Kamath H, Tarascon J-M (2011) Electrical energy storage for the grid: a battery of choices. Science 334(80):928–935. doi:10.1126/science.1212741
Thackeray MM, Wolverton C, Isaacs ED (2012) Electrical energy storage for transportation—approaching the limits of, and going beyond, lithium–ion batteries. Energy Environ Sci 5:7854. doi:10.1039/c2ee21892e
Nataf K, Bradley TH (2016) An economic comparison of battery energy storage to conventional energy efficiency technologies in Colorado manufacturing facilities. Appl Energy 164:133–139. doi:10.1016/j.apenergy.2015.11.102
Parra D, Patel MK (2016) Effect of tariffs on the performance and economic benefits of PV-coupled battery systems. Appl Energy 164:175–187. doi:10.1016/j.apenergy.2015.11.037
Ma T, Yang H, Lu L (2015) Development of hybrid battery–supercapacitor energy storage for remote area renewable energy systems. Appl Energy 153:56–62. doi:10.1016/j.apenergy.2014.12.008
Cho J, Jeong S, Kim Y (2015) Commercial and research battery technologies for electrical energy storage applications. Prog Energy Combust Sci 48:84. doi:10.1016/j.pecs.2015.01.002
Biswal A, Tripathy C, Sanjay K (2015) RSC advances perspective on worldwide production, reserves and its role in electrochemistry. RSC Adv 5:58255–58283. doi:10.1039/C5RA05892A
Ingale ND, Gallaway JW, Nyce M et al (2015) Rechargeability and economic aspects of alkaline zinc e manganese dioxide cells for electrical storage and load leveling. J Power Sources 276:7–18. doi:10.1016/j.jpowsour.2014.11.010
Bhadra S, Hsieh AG, Wang MJ et al (2016) Anode characterization in zinc–manganese dioxide AA alkaline batteries using electrochemical-acoustic time-of-flight analysis. J Electrochem Soc 163:1050–1056. doi:10.1149/2.1201606jes
Rus ED, Moon D, Bai J et al (2016) Electrochemical behavior of electrolytic manganese dioxide in aqueous KOH and LiOH solutions: a comparative study. J Electrochem Soc 163:356–363. doi:10.1149/2.1011602jes
Kim SU, Monroe CW (2013) Increasing the rate capability of batteries with electrolyte flow. Appl Energy 103:207–211. doi:10.1016/j.apenergy.2012.09.028
Devenney M, Donne SW, Gorer S (2004) Application of combinatorial methodologies to the synthesis and characterization of electrolytic manganese dioxide. J Appl Electrochem 34:643–651. doi:10.1023/B:JACH.0000021915.73788.4c
Stani A, Taucher-Mautner W, Kordesch K, Daniel-Ivad J (2006) Development of flat plate rechargeable alkaline manganese dioxide–zinc cells. J Power Sources 153:405–412. doi:10.1016/j.jpowsour.2005.05.031
Scherson D, Division C (1997) In situ X-ray absorption fine structure studies of a manganese dioxide electrode in a rechargeable MnO2/Zn alkaline battery environment. J Electrochem Soc 144:1598–1603
Kozawa A, Powers RA (1966) The manganese dioxide electrode in alkaline electrolyte; the electron-proton mechanism for the discharge process from MnO2 to MnO1.5. J Electrochem Soc 113:870. doi:10.1149/1.2424145
Bonakdarpour A, Mehta S, Xi W, Afonso G, Wilkinson D, Impact of BaSO4 additive on the cycling performance of MnO2/Zn alkaline batteries (article in preparation)
Kannan AM, Bhavaraju S, Prado F et al (2002) Characterization of the bismuth-modified manganese dioxide cathodes in rechargeable alkaline cells. J Electrochem Soc 149:A483. doi:10.1149/1.1459713
Daniel-Ivad J (2009) Zinc–manganese. Encycl Electrochem Power Sources 5:497–512
Malloy AP, Donne SW (2008) Porosity changes during reduction of γ-MnO2 for aqueous alkaline applications. J Electrochem Soc 155:A817. doi:10.1149/1.2971193
Minakshi M, Singh P, Carter M, Prince K (2008) The Zn–MnO2 battery: the influence of aqueous LiOH and KOH electrolytes on the intercalation mechanism. Electrochem Solid State Lett 11:A145. doi:10.1149/1.2932056
Bailey MR, Donne SW (2012) The effect of barium hydroxide on the rechargeable performance of alkaline-MnO2. J Electrochem Soc 159:A999–A1004. doi:10.1149/2.047207jes
Gilman S, Mansfeld F (1970) The effect of several electrode and electrolyte additives on the corrosion and polarization behavior of the alkaline zinc electrode. J Electrochem Soc 1:1328–1333. doi:10.1149/1.2407303
Mondoloni C (1992) Rechargeable alkaline manganese dioxide batteries. J Electrochem Soc 139:954. doi:10.1149/1.2069374
Shen Y, Kordesch K (2000) Mechanism of capacity fade of rechargeable alkaline manganese dioxide zinc cells. J Power Sources 87:162–166. doi:10.1016/S0378-7753(99)00476-0
Nevers DR, Peterson SW, Robertson L et al (2014) The effect of carbon additives on the microstructure and conductivity of alkaline battery cathodes. J Electrochem Soc 161:A1691–A1697. doi:10.1149/2.0771410jes
Bailey MR, Donne SW (2011) Electrochemical impedance spectroscopy study into the effect of titanium dioxide added to the alkaline manganese dioxide cathode. J Electrochem Soc 158:A802. doi:10.1149/1.3586045
Bailey MR, Denman JA, King BV, Donne SW (2012) Role of titanium dioxide in enhancing the performance of the alkaline manganese dioxide cathode. J Electrochem Soc 159:A158. doi:10.1149/2.080202jes
Raghuveer V, Manthiram A (2005) Effect of BaBiO3 and Ba0.6K0.4BiO3 additives on the rechargeability of manganese oxide cathodes in alkaline cells. Electrochem Commun 7:1329–1332. doi:10.1016/j.elecom.2005.09.012
Hertzberg B, Sviridov L, Stach EA et al (2014) A manganese-doped barium carbonate cathode for alkaline batteries. J Electrochem Soc 161:A835–A840. doi:10.1149/2.083405jes
Minakshi M (2011) Alkaline-earth oxide modified MnO2 cathode: enhanced performance in an aqueous rechargeable battery. Ind Eng Chem Res 50:8792–8795. doi:10.1021/ie2001742
Kordesch K, Gsellmann J (1984) Production of electrolytic manganese dioxide for alkaline cells. German Patent 3,337,568
Taucher W, Kordesch K, Daniel-Ivad J (1992) Cathodes for zinc manganese dioxide cells having barium additives. WO 93/12551
Daniel-Ivad J, Daniel-Ivad E, Book JR (2002) Additives for rechargeable alkaline manganese dioxide zinc cells. US Patent 6,361,899
Balachandran D, Morgan D, Ceder G (2002) First principles study of H-insertion in MnO2. J Solid State Chem 166:91–103. doi:10.1006/jssc.2002.9564
Roberge PR, Farahani M, Tomantschger K, Oran E (1994) Electrochemical characterization of flat-plate rechargeable alkaline manganese dioxide–zinc cells. J Power Sources 47:13–26. doi:10.1016/0378-7753(94)80046-4
Zheng Y, Wang JM, Chen H et al (2004) Effects of barium on the performance of secondary alkaline zinc electrode. Mater Chem Phys 84:99–106. doi:10.1016/j.matchemphys.2003.11.015
Reddy T (2010) Linden’s handbook of batteries, 4th edn. McGraw-Hill, New York
Raghuveer V, Manthiram A (2006) Role of TiB2 and Bi2O3 additives on the rechargeability of MnO2 in alkaline cells. J Power Sources 163:598–603. doi:10.1016/j.jpowsour.2006.09.029
Acknowledgements
The authors would like to acknowledge the National Science and Engineering Research Council of Canada for funding through a CRD (CRDPJ 418933) grant and OTI Inc. for financial support and Mr. Bill Coote for useful discussions.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Mehta, S.A., Bonakdarpour, A. & Wilkinson, D.P. Impact of cathode additives on the cycling performance of rechargeable alkaline manganese dioxide–zinc batteries for energy storage applications. J Appl Electrochem 47, 167–181 (2017). https://doi.org/10.1007/s10800-016-1034-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-016-1034-1