Abstract
The assumption of electrochemical equilibrium at membrane–electrolyte interfaces is frequently accepted in a mathematical simulation of multiple ion transport (MIT) across a single-layer perfluorinated sulfonated cation-selective membrane (CM). This assumption is obviously inaccurate at high electric current loads typical of industrial applications, e.g. brine electrolysis. An assessment of this problem is one of the main objectives of this contribution. For this purpose, a one-dimensional stationary Poisson–Nernst–Planck (PNP) model was employed to describe MIT across a CM. The model input parameters used correspond closely to industrial chlor-alkali electrolysis. The model results are compared with those of an equivalent model published earlier that considered the classical Nernst–Planck equation and Donnan equilibrium at the CM–electrolyte interfaces (denoted as the DNP model). Both the DNP and the PNP models provide identical results at low current loads. However, a comparison at high current loads close to ‘industrial scale’ was impossible due to convergence problems of the DNP model. The ion transport numbers and membrane permselectivity were estimated by means of the PNP model. This model predicts conditions at the membrane interfaces close to thermodynamic equilibrium even in a current density range up to 10,000 A m−2. Additionally, the PNP model takes into account the kinetics of water autoprotolysis. It was shown that a high flux of OH− ions across a CM effectively alkalizes the catholyte diffusion layer, ensuring a precipitation of alkaline earth cations outside the CM and thus minimizing internal CM blockage.
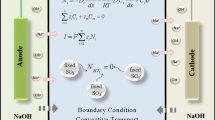
Graphical abstract
















Similar content being viewed by others
Abbreviations
- c :
-
Molar concentration (mol m−3)
- D :
-
Diffusion coefficient (m2 s−1)
- F :
-
Faraday’s constant (F = 96484.56 C mol−1)
- J :
-
Molar flux density (mol m−2 s−1)
- j :
-
Local current density (A m−2)
- k :
-
Hydraulic membrane permeability (m2)
- k b , k f :
-
Kinetic rate constant of backward and forward reaction (m3 mol−1 s−1)
- K w :
-
Water autoprotolysis equilibrium constant (K w = 1 × 10−8 mol2 m−6)
- N :
-
Number of ions included in the system
- p :
-
Hydrostatic pressure (Pa)
- q :
-
Space charge density (C m−3)
- R :
-
Universal gas constant (R = 8.314 J K−1 mol−1)
- S :
-
Source term (mol m−3 s−1)
- t :
-
Transport number
- T :
-
Absolute temperature (K)
- U :
-
Voltage (V)
- v :
-
Convective velocity (m s−1)
- w :
-
Width (m)
- x :
-
Coordinate (m)
- z :
-
Valence number
- ε r :
-
Relative permittivity of free water (ε r = 78.5)
- ε 0 :
-
Permittivity of vacuum (ε 0 = 8.8542 × 10−10 F m−1)
- η :
-
Dynamic viscosity (kg m−1 s−1)
- ϕ :
-
Electric potential (V)
- σ :
-
Conductivity (S m−1)
- ADL:
-
Anodic diffusion layer
- CDL:
-
Cathodic diffusion layer
- CM:
-
Cation-selective membrane
- DER:
-
Donnan exclusion region
- fix:
-
Fixed charge
- i:
-
Ion
- p:
-
Phase
- ba:
-
Bulk anolyte
- bc:
-
Bulk catholyte
- p:
-
Phase
- 1D:
-
One-dimensional
- ADL:
-
Anodic diffusion layer
- CDL:
-
Cathodic diffusion layer
- CM:
-
Cation-selective membrane
- DER:
-
Donnan exclusion region
- DNP:
-
Donnan–Nernst–Planck model
- DNP1, DNP2:
-
Donnan equilibrium Nernst–Planck model 1 and 2
- ISM:
-
Ion-selective membrane
- MIT:
-
Multiple ion transport
- PDE:
-
Partial differential equation
- PNP:
-
Poisson–Nernst–Planck model
- WSR:
-
Water splitting/recombination reaction
References
Wirguin CH (1996) Recent advance in perfluorinated ionomer membranes: structure, properties and applications. J Mem Sci 120:1–33
Di Noto V, Lavina S, Giffin GA, Negro E, Scrosati B (2011) Editorial, polymer electrolytes: present, past and future. Electrochim Acta 57:4–13
Nagarale RK (2006) Recent developments on ion-exchange membranes and electro-membrane processes. Adv Colloid Interface Sci 119:97–130
Strathmann H (2010) Electrodialysis, a mature technology with a multitude of new applications. Desalination 264(3):268–288
Kodym R, Panek P, Snita D, Tvrznik D, Bouzek K (2012) Macrohomogeneous approach to a two-dimensional mathematical model of an industrial-scale electrodialysis unit. J Appl Electrochem 42:645–666
Kodým R, Drakselová M, Pánek P, Němeček M, Šnita D, Bouzek K (2015) Novel approach to mathematical modeling of the complex electrochemical systems with multiple phase interfaces. Electrochim Acta 179:538–555
Panek P, Kodym R, Snita D, Bouzek K (2015) Spatially two-dimensional mathematical model of the flow hydrodynamics in a spacer-filled channel—the effect of inertial forces. J Mem Sci 492:588–599
Lu J, Wang Y, Lu Y, Wang G, Kong L, Zhu J (2010) Numerical simulation of the electrodeionization (EDI) process for producing ultrapure water. Electrochim Acta 55:7188–7198
Pletcher D, Walsh FC (1990) Industrial electrochemistry. University Press, Cambridge
Secanell M, Wishart J, Dobson P (2011) Computational design and optimization of fuel cells and fuel cell systems: a review. J Power Sources 196:3690–3704
Hnát J, Paidar M, Schauer J, Žitka J, Bouzek K (2011) Polymer anion selective membranes for electrolytic splitting of water. Part I: stability of ion-exchange groups and impact of the polymer binder. J Appl Electrochem 41:1043–1052
Hnát J, Paidar M, Schauer J, Žitka J, Bouzek K (2012) Polymer anion-selective membranes for electrolytic splitting of water. Part II: enhancement of ionic conductivity and performance under conditions of alkaline water electrolysis (2012). J Appl Electrochem 42:545–554
Wang W, Li L, Li B, Wei X, Li L, Yang Z (2013) Recent progress in redox flow battery research development. Adv Funct Mater 23:970–986
Veerman J, Saakes M, Metz SJ, Harmsen GJ (2011) Reverse electrodialysis: a validated process model for design and optimization. Chem Eng J 166:256–268
Verbrugge MW, Hill RF (1992) Measurement of ionic concentration profiles in membranes during transport. Electrochim Acta 37:221
Buck RP (1984) Kinetics of bulk and interfacial ionic motion: microscopic bases and limits for the Nernst–Planck equation applied to membrane systems. J Membr Sci 17:1–62
van der Stegen JHG, van der Veen AJ, Weerdenburg H, Hogendoorn JA, Versteeg GF (1999) Application of the Maxwell-Stefan theory to the transport in ion-selective membranes used in the chlor–alkali electrolysis process. Chem Eng Sci 54:2501–2511
Hogendoorn JA, van der Veen AJ, van der Stegen JHG, Kuipers JAM, Versteeg GF (2001) Application of the Maxwell–Stefan theory to the membrane electrolysis process model development and simulations. Comput Chem Eng 25:1251–1265
Fila V, Bouzek K (2003) A mathematical model of multiple ion transport across an ion-selective membrane under current load conditions. J Appl Electrochem 33:675–684
Fila V, Bouzek K (2008) The effect of convection in the external diffusion layer on the results of a mathematical model of multiple ion transport across an ion-selective membrane. J Appl Electrochem 38:1241–1252
Manzanares JA, Murphy WD, Mafe S, Reiss H (1993) Numerical simulation of the nonequilibrium diffuse double layer in ion-exchange membranes. J Phys Chem 97:8524–8530
Volgin VM, Davydov AD (2005) Ionic transport through ion-exchange and bipolar membranes. J Membr Sci 259:110–121
van der Stegen JHG, Weerdenburg H, van der Veen AJ, Hogendoorn JA, Versteeg GF (1999) Application of the Pitzer model for the estimation of activity coefficients of electrolytes in ion selective membranes. Fluid Phase Equilib 157:181–196
Kontturi K, Murtomäki L, Manzanares JA (2008) Ionic transport processes in electrochemistry and membrane science, 1st edn. Oxford University Press, Oxford
Cwirko EH, Carbonell RG (1992) Ionic equilibria in ion-exchange membranes: a comparison of pore model predictions with experimental results. J Membr Sci 67:227
Šnita D, Pačes M, Lindner J, Kosek J, Marek M (2001) Nonlinear behaviour of simple ionic systems in hydrogel in an electric field. Faraday Discuss 120:53–66
Přibyl M, Šnita D, Kubíček M (2006) Adaptive mesh simulations of ionic systems in micro-capillaries based on the estimation of transport times. Comput Chem Eng 30:674
Lindner J, Šnita D, Marek M (2002) Modeling of ionic systems with a narrow acid–base boundary. Phys Chem Chem Phys 4:1348
Van Parys H, Telias G, Nedashkivskyi V, Mollayb B, Vandendael I, Van Damme S, Deconinck J, Hubin A (2010) On the modeling of electrochemical systems with simultaneous gas evolution. Case study: the zinc deposition mechanism. Electrochim Acta 55:5709–5718
Danielsson CO, Dahlkild A, Velin A, Behm M (2009) A model for the enhanced water dissociation on monopolar membranes. Electrochim Acta 54:2983–2991
Jialin L, Yazhen W, Changying Y, Guangdou L, Hong S (1998) Membrane catalytic deprotonation effects. J Membr Sci 147:247–256
Moore WJ (1963) Physical chemistry, 4th edn. Longmans Green & Co. Ltd, London
Onsanger L (1934) Deviations from Ohm’s law in weak electrolytes. J Chem Phys 2:512–599
Xu T (2002) Effect of asymmetry in a bipolar membrane on water dissociation—a mathematical analysis. Desalination 150:65–74
Balster J, Srinkantharajah S, Sumbharaju S, Pünt I, Lammertink RGH, Stamatialis DF, Wessling M (2010) Tailoring the interface layer of the bipolar membrane. J Membr Sci 365(1–2):386–398
Simons R (1985) Water splitting in ion exchange membranes. Electrochim Acta 30:275
Schlögl R (1966) Membrane permeation in systems far from equilibrium. Ber Bunsenges Phys Chem 70:400
Verbrugge MW, Hill RF (1990) Ion and solvent transport in ion-exchange membranes I: a macrohomogeneous mathematical model. J Electrochem Soc 137(3):886–893
Verbrugge MW, Hill RF (1990) Transport phenomena in perfluorosulfonic acid membrane during the passage of current. J Electrochem Soc 137(4):1131–1138
Liang YY, Fimbres Weihs GA, Wiley DE (2014) Approximation for modelling electro-osmotic mixing in the boundary layer of membrane systems. J Membr Sci 450:18–27
Natzlet WC, Moore CB (1985) Recombination of H+ and OH− In pure liquid water. J Phys Chem 89:2605–2612
Cwirko EH, Carbonell RG (1992) Interpretation of transport coefficients in Nafion using a parallel pore model. J Membr Sci 67:227–247
Gur Y, Ravina I, Babchin A (1978) On the electrical double layer theory. Part II. The Poisson–Boltzmann equation including hydration forces. J Colloid Interface Sci 64:333
Guzmán-Garcia AG, Pintauro PN, Verbrugge MW, Hill RF (1990) Development of a space charge transport model for ion-exchange membranes. AIChE J 36(7):1061–1074
Booth F (1951) The dielectric constant of water and the saturation effect. J Chem Phys 19:391
Koter S, Narebska A (1987) Conductivity of ion exchange membranes II mobilities of ions and water. Electrochim Acta 32(3):455–458
Narebska A, Koter S (1987) Conductivity of ion exchange membranes I convection conductivity and other components. Electrochim Acta 32(3):449–453
Acknowledgments
The authors gratefully acknowledge the financial aid for this research by the Science Foundation (GACR) of the Czech Republic, Project No: 14-17351P.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kodým, R., Fíla, V., Šnita, D. et al. Poisson–Nernst–Planck model of multiple ion transport across an ion-selective membrane under conditions close to chlor-alkali electrolysis. J Appl Electrochem 46, 679–694 (2016). https://doi.org/10.1007/s10800-016-0945-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-016-0945-1