Abstract
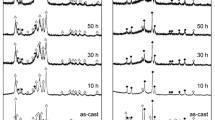
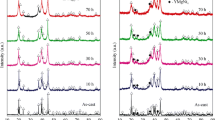
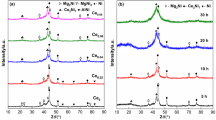
Mg in Mg2Ni-type alloy was partially substituted with Y to improve the electrochemical performance of the alloy. The as-cast Mg2Ni-type Mg20−x Y x Ni10 (x = 0, 1, 2, 3, 4) electrode alloys were prepared by vacuum induction melting under the protection of high-purity helium atmosphere and subsequent mechanical milling for different time points. Effects of Y substitution with Mg on the microstructures and electrochemical performance of the as-cast and milled alloys were investigated in detail by X-ray diffraction, transmission electron microscopy, scanning electron microscopy coupled with energy-dispersive spectrometry, and automatic galvanostatic system. Results revealed that the substitution of Y with Mg evidently changed the phase composition of alloys. At a Y content of x ≤ 1, the major phase in alloys was Mg2Ni, but the phase changed to YMgNi4 + YMg3 with further addition of Y content. Electrochemical measurement showed that variations in the discharge capacity of alloys with Y content were closely related to the milling time. At ≤ 10-h milling time, the discharge capacity of alloy invariably increased with the increasing Y content; at > 10-h milling time, the discharge capacity initially increased, and then decreased with the increasing Y content. The substitution of Y with Mg dramatically ameliorated the cycle stability of the as-cast and milled alloys. Furthermore, high rate of discharge ability, electrochemical impedance spectrum, Tafel polarization curves, and potential-step measurements indicated that the electrochemical kinetic properties of the as-cast and milled alloys initially increased and then decreased with the increasing Y content.













Similar content being viewed by others
References
Jia Y, Sun CH, Shen SH, Zou J, Mao SS, Yao XD (2015) Combination of nanosizing and interfacial effect: future perspective for designing Mg-based nanomaterials for hydrogen storage. Renew Sustain Energy Rev 44:289–303
Jain IP, Lal C, Jain A (2010) Hydrogen storage in Mg: a most promising material. Int J Hydrog Energy 35:5133–5144
Mori D, Hirose K (2009) Recent challenges of hydrogen storage technologies for fuel cell vehicles. Int J Hydrog Energy 34:4569–4574
Ebrahimi-Purkani A, Kashani-Bozorg SF (2008) Nanocrystalline Mg2Ni-based powders produced by high-energy ball milling and subsequent annealing. J Alloys Compd 456:211–215
Atias-Adrian IC, Deorsola FA, Ortigoza-Villalba GA, DeBenedetti B, Baricco M (2011) Development of nanostructured Mg2Ni alloys for hydrogen storage applications. Int J Hydrog Energy 36:7897–7901
Ohara R, Lan CH, Hwang CS (2013) Electrochemical and structural characterization of electroless nickelcoating on Mg2Ni hydrogen storage alloy. J Alloys Compd 580:S368–S372
Xie DH, Li P, Zeng CX, Sun JW, Qu XH (2009) Effect of substitution of Nd for Mg on the hydrogen storage properties of Mg2Ni alloy. J Alloys Compd 478:96–102
Zhao X, Han SM, Zhu Y, Chen XC, Ke DD, Wang ZB, Liu T, Ma YF (2015) Investigation on hydrogenation performance of Mg2Ni + 10 wt% NbN composite. J Solid State Chem 221:441–444
Redzeb M, Zlatanova Z, Spassov T (2013) Influence of boron on the hydriding of nanocrystalline Mg2Ni. Intermetallics 34:63–68
Lass EA (2011) Hydrogen storage measurements in novel Mg-based nanostructured alloys produced via rapid solidification and devitrification. Int J Hydrog Energy 36:10787–10796
Kou HC, Hou XJ, Zhang TB, Hu R, Li JS, Xue XY (2013) On the amorphization behavior and hydrogenation performance of high-energy ball-milled Mg2Ni alloys. Mater Charact 80:21–27
Teresiak A, Gebert A, Savyak M, Uhlemann M, Mickel C, Mattern N (2005) In situ high temperature XRD studies of the thermal behaviour of the rapidly quenched Mg77Ni18Y5 alloy under hydrogen. J Alloys Compd 398:156–164
Drenchev N, Spassov T, Bliznakov S (2008) Influence of tin on the electrochemical and gas phase hydrogen sorption in Mg2−x Sn x Ni (x = 0, 0.1, 0.3). J Alloys Compd 450:288–292
Dornheim M, Doppiu S, Barkhordarian G, Boesenberg U, Klassen T, Gutfleisch O, Bormann R (2007) Hydrogen storage in magnesium-based hydrides and hydride composites. Scr Mater 56:841–846
Zhou NG, Ju DY (2014) Study on preparation and properties evaluation of Mg/Ni/Ti hydrogen storage material. Int J Hydrog Energy 39:19630–19636
Li X, Yang T, Zhang YH, Zhao DL, Ren HP (2014) Kinetic properties of La2Mg17−x wt% Ni (x = 0–200) hydrogen storage alloys prepared by ball milling. Int J Hydrog Energy 39:13557–13563
Aono K, Orimo S, Fujii H (2000) Structural and hydriding properties of MgYNi4: a new intermetallic compound with C15b-type Laves phase structure. J Alloys Compd 309:L1–L4
Zhang YH, Li C, Cai Y, Hu F, Liu ZC, Guo SH (2014) Highly improved electrochemical hydrogen storage performances of the Nd–Cu–added Mg2Ni-type alloys by melt spinning. J Alloys Compd 584:81–86
Zhang YH, Zhao C, Yang T, Shang HW, Xu C, Zhao DL (2013) Comparative study of electrochemical performances of the as-melt Mg20Ni10−xMx (M = None, Cu Co, Mn; x = 0, 4) alloys applied to Ni/metal hydride (MH) battery. J Alloys Compd 555:131–137
Ren HP, Zhang YH, Li BW, Zhao DL, Guo SH, Wang XL (2009) Influence of the substitution of La for Mg on the microstructure and hydrogen storage characteristics of Mg20−x La x Ni10 (x = 0–6) alloys. Int J Hydrog Energy 34:1429–1436
Lenain C, Aymard L, Dupont L, Tarascon JM (1999) A new Mg0.9Y0.1Ni hydride forming composition obtained by mechanical grinding. J Alloys Compd 292:84–89
Pan HG, Chen N, Gao MX, Li R, Lei YQ, Wang QD (2005) Effects of annealing temperature on structure and the electrochemical properties of La0.7Mg0.3Ni2.45Co0.75Mn0.1Al0.2 hydrogen storage alloy. J Alloys Compd 397:306–312
Zheng G, Popov BN, White RE (1995) Electrochemical determination of the diffusion coefficient of hydrogen through an LaNi4.25Al0.75 electrode in alkaline aqueous solution. J Electrochem Soc 142:2695–2698
Feng F, Northwood DO (2004) Hydrogen diffusion in the anode of Ni/MH secondary batteries. J Power Sources 136:346–350
Kalinichenka S, Röntzsch L, Riedl T, Weißgärber T, Kieback B (2011) Hydrogen storage properties and microstructure of melt-spun Mg90Ni8RE2 (RE = Y, Nd, Gd). Int J Hydrog Energy 36:10808–10815
Cui N, Luo JL (1999) Electrochemical study of hydrogen diffusion behavior in Mg2Ni-type hydrogen storage alloy electrodes. Int J Hydrog Energy 24:37–42
Ratnakumar BV, Witham C, Bowman RC Jr, Hightower A, Fultz B (1996) Electrochemical studies on LaNi5−x Sn x metal hydride alloys. J Electrochem Soc 143:2578–2584
Zhao XY, Ding Y, Ma LQ, Wang LY, Yang M, Shen XD (2008) Electrochemical properties of MmNi3.8Co0.75Mn0.4Al0.2 hydrogen storage alloy modified with nanocrystalline nickel. Int J Hydrog Energy 33:6727–6733
Kuriyama N, Sakai T, Miyamura H, Uehara I, Ishikawa H, Iwasaki T (1993) Electrochemical impedance and deterioration behavior of metal hydride electrodes. J Alloys Compd 202:183–197
Kleperis J, Wójcik G, Czerwinski A, Skowronski J, Kopczyk M, Beltowska-Brzezinska M (2001) Electrochemical behavior of metal hydrides. J Solid State Electrochem 5:229–249
Nobuhara K, Kasai H, Diño WA, Nakanishi H (2004) H2 dissociative adsorption on Mg, Ti, Ni, Pd and La surfaces. Surf Sci 566–568:703–707
Zhang YH, Li BW, Ren HP, Cai Y, Dong XP, Wang XL (2008) Cycle stabilities of the La0.7Mg0.3Ni2.55−x Co0.45M x (M = Fe, Mn, Al; x = 0, 0.1) electrode alloys prepared by casting and rapid quenching. J Alloy Compd 458:340–345
Acknowledgments
This study was financially supported by the National Natural Science Foundations of China (51161015 and 51371094).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Y., Zhai, T., Yang, T. et al. Electrochemical hydrogen-storage performance of Mg20−x Y x Ni10 (x = 0–4) alloys prepared by mechanical milling. J Appl Electrochem 45, 931–941 (2015). https://doi.org/10.1007/s10800-015-0861-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-015-0861-9