Abstract
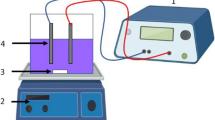
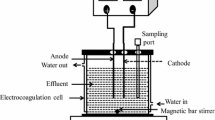
In this work, the Box–Behnken experimental design and the surface response methodology were applied for the optimization of the operational conditions of the electro-catalytic degradation of wastewaters, resulting from a local textile industry. The experiments were carried out in a laboratory scale batch cell reactor, with monopolar configuration, and electrodes made of boron-doped diamond (anode) and titanium (cathode). The multifactorial experimental design included the following variables: current density (i: 5–10 mA/cm2), pH (3–7), and submerged cathode area (CA: 8–24 cm2). To determine the process efficiency, the degradation percentage of: the chemical oxygen demand (%DCOD), the total organic carbon (%DTOC) and the color (%DC) were defined as response variables. The following optimal conditions for the electro-oxidation (EO) process were obtained: i = 10 mA/cm2, pH = 3 and CA = 16 cm2, reaching ca. 92 % of DC, 37 % of DCOD and 31 % of DTOC. The electro-Fenton (EF) and photo-electro-Fenton (PEF) processes were also evaluated at EO optimal conditions. For the EF process, with addition of iron (0.3 mM), the %DC, %DCOD and %DTOC was enhanced to 95, 52 and 45 %, respectively. For the PEF process (UV = 365 nm), it was possible to reach 98 %DC, 56 %DCOD and 48 %DTOC.






Similar content being viewed by others
References
US EPA 2011–US Environmental Protection Agency 2011. Available in: www.epa.gov
Villanueva-Rodríguez M, Hernández-Ramírez A, Peralta-Hernández JM, Bandala ER, Quiroz-Alfaro MA (2009) Enhancing the electrochemical oxidation of Acid-Yellow 36 azo dye using boron-doped diamond electrodes by addition of ferrous ion. J Hazard Mater 167:1226–1230
Ramírez C, Saldaña A, Hernández B, Acero R, Guerra R, Garcia-Segura S, Brillas E, Peralta-Hernández J (2012) Electrochemical oxidation of methyl orange azo dye at pilot flow plant using BDD technology. J Ind Eng Chem 19:571–579
GilPavas E, Dobrosz-Gómez I, Gómez-García MA (2012) Decolorization and mineralization of Diarylide Yellow 12 (PY12) by photo-Fenton process: the response surface methodology as the optimization tool. Water Sci Technol 65:1795–1800
Konstantinou IK, Albanis TA (2004) TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: kinetic and mechanistic investigation. A review. Appl Catal B: Environ 49:1–14
Ruiz J, Arias C, Brillas E, Hernández-Ramírez A, Peralta-Hernández JM (2011) Mineralization of Acid Yellow 36 azo dye by electro-Fenton and solar photoelectro-Fenton processes with a boron-doped diamond anode. Chemosphere 82:495–501
Bandala ER, Pelaez MA, García AJ, Salgado MJ, Moeller G (2008) Photocatalytic decolorization of synthetic and real textile wastewater containing benzidine based azo dyes. Chem Eng Process 47:169–176
Chacón JM, Leal MT, Sanchez M, Bandala ER (2006) Solar photocatalytic degradation of azo-dyes by photo-Fenton process. Dyes Pigments 69:144–150
Parsons S (ed) (2004) Advanced oxidation processes for water and wastewater treatment. IWA Publishing, London
Maciel R, Sant’Anna GL Jr, Dezotti M (2004) Phenol removal from high salinity effluents using Fenton’s reagent and photo-Fenton reactions. Chemosphere 57:711–719
Xiao Q, Si Z, Zhang J, Xiao C, Tan X (2008) Photoinduced hydroxyl radical and photocatalytic activity of samarium-doped TiO2 nanocrystalline. J Hazard Mater 150:62–67
Yavuz Y, Shahbazi R (2012) Anodic oxidation of Reactive Black 5 dye using boron doped diamond anodes in a bipolar trickle tower reactor. Sep Purif Technol 85:130–136
Cruz K, Torres O, García A, Brillas E, Hernández A, Peralta JM (2012) Optimization of electro-Fenton/BDD process for decolorization of a model azo dye wastewater by means of response surface methodology. Desalination 286:63–68
Cruz K, Torres O, García A, Guzmán JL, Reyes LH, Hernández A, Peralta JM (2010) Determination of optimum operating parameters for Acid Yellow 36 decolorization by electro-Fenton process using BDD cathode. Chem Eng J 160:199–206
Brillas E, Baños MA, Garrido JA (2003) Mineralization of herbicide 3, 6-dichloro-2-methoxybenzoic acid in aqueous medium by anodic oxidation, electro-Fenton and photoelectro-Fenton. Electrochim Acta 48:1697–1705
Moreira FC, Garcia-Segura S, Vilar JP, Boaventura AR, Brillas E (2013) Decolorization and mineralization of Sunset Yellow FCF azo dye by anodic oxidation, electro-Fenton, UVA photoelectro-Fenton and solar photoelectro-Fenton processes. Appl Catal B: Environ 142–143:877–890
Alves S, Ferreira T, Sabatini N, Trientini A, Migliorini F, Baldan M, Ferreira N, Lanza M (2012) A comparative study of the electrochemical oxidation of the herbicide tebuthiuron using boron-doped diamond electrodes. Chemosphere 88:155–160
Yavuz Y, Koparal S, Ogutveren U (2010) Treatment of petroleum refinery wastewater by electrochemical methods. Desalination 258:201–205
GilPavas E, Betancourt A, Ángulo M, Dobrosz-Gómez I, Gómez-García MA (2009) The Box–Behnken experimental design for the optimization of the electrocatalytic treatment of wastewaters with high concentrations of phenol and organic matter. Water Sci Technol 60:2809–2818
American Public Health Association (APHA) (2005) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association (APHA), Washington Centennial Edition
Gogate PR, Pandit AB (2004) A review of imperative technologies for wastewater treatment I: oxidation technologies at ambient conditions. Adv Environ Res 8:501–551
Montgomery DC (2010) Design and analysis of experiments, 8th edn. Wiley, Hoboken
Zarei M, Niaei A, Salari D, Khataee A (2010) Application of response surface methodology for optimization of peroxi-coagulation of textile dye solution using carbon nanotube-PTFE cathode. J Hazard Mater 173:544–551
Panizza M, Cerisola G (2005) Application of diamond electrodes to electrochemical processes. Electrochim Acta 51:191–199
Acknowledgments
The authors thank to the Dirección de Investigación de la Universidad EAFIT, Medellin-Colombia and COLCIENCIAS, Young Researchers Program, for financial support of this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
GilPavas, E., Medina, J., Dobrosz-Gómez, I. et al. Statistical optimization of industrial textile wastewater treatment by electrochemical methods. J Appl Electrochem 44, 1421–1430 (2014). https://doi.org/10.1007/s10800-014-0767-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-014-0767-y