Abstract
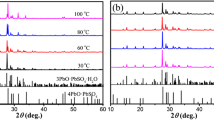
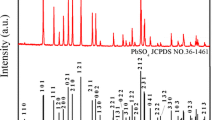
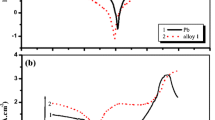
Acid absorption is a routine analytical method used in the manufacturing of lead oxide, that is, then further used to manufacture Pb-acid battery electrodes. This study had a new look at the definition of acid absorption being an indication of the acid reactivity of the oxide forming certain lead sulphate-related phases. Quantitative powder X-ray diffraction analysis of the acid reaction products showed there were significant differences in the phases formed that could be related to the amount of acid used in the acid absorption test. The study also showed that there were significant changes in the surface area of the oxides once they had reacted with the acid, where a traditionally slow reacting oxide such as β-PbO would show the greatest increase in material surface area once reacted with the acid. The accuracy of the method used by various laboratories was also studied by comparing the results obtained from two different methods and from three different laboratories. The results showed that there were significant differences between the reported values, and that one should with caution compare acid absorption numbers obtained from different laboratories.









Similar content being viewed by others
References
Vinal GW (1955) Storage batteries, 4th edn. Wiley, New York, p 24
Prout L (1994) J Power Sources 47:197–217
Heubach R (1981) The Battery Man 23(4):3–9
Mayer MG, Rand DAJ (1996) J Power Sources 59:17–24
Brockmann KH (1989) J Power Sources 28:121–125
Shin JH, Kim KW, Ahn HJ (2000) J Power Sources 89:46–51
Rand DA (1989) J Power Sources 28:107–111
Hardy D, Marx R (1992) J Power Sources 38:75–85
Corino GL, Hill RJ, Jessel AM, Rand DAJ, Wunderlich JA (1985) J Power Sources 16:141–168
Bruker AXS (2005): Topas V3: general profile and structure analysis software for powder diffraction data. Bruker AXS, Karlsruhe, Germany
Steele IM, Pluth JJ, Richardson JW (1997) J Solid State Chem 132:173–181
Garnier P, Moreau J, Gavarri JR (1990) Mat Res Bull 25:979–986
Klug HP (1946) J Am Chem Soc 68:1493–1494
Mentzen BF, Latrach A, Bouix J, Hewat AW (1984) Mat Res Bull 19:549–554
Andreev YG (1994) J Appl Cryst 27:288–297
Morgan E (1991) Chemometrics: experimental design. Wiley, New York, pp 4–12
Dimitrov M, Pavlov D, Rogachev T, Matrakova M, Bogdanova L (2005) J Power Sources 140:168–180
Acknowledgments
The authors thank Willard Batteries and First National Batteries for supplying the various manufactured lead oxide samples. The authors also thank the South African NRF for their financial contribution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ferg, E.E., Phangalala, T. & van Dyl, T. A new look at determining acid absorption of lead oxide used in the manufacturing of Pb-acid batteries. J Appl Electrochem 40, 383–391 (2010). https://doi.org/10.1007/s10800-009-0007-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-009-0007-z