Abstract
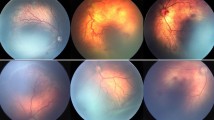
This study aims to present five cases with retinopathy of prematurity (ROP) who were found to have intraocular air bubbles after intravitreal injection (IVI) treatment. The medical records of 148 infants who underwent IVI for ROP were retrospectively reviewed and the ones who demonstrated post-injection intraocular air bubble formation were recruited. Of the 148 patients (31 babies received ranibizumab, 20 babies received aflibercept, 97 babies received bevacizumab), five were found to have intraocular air bubbles right after the IVI. Two infants received intravitreal ranibizumab and three received intravitreal bevacizumab injections. Although intraocular pressure increased temporarily, no intraocular sterile or infective reactions were observed in the postoperative period. The air bubble was found to resorb spontaneously within 72 h. The occurrence rate of the intravitreal air bubbles in our series was 3.37 % despite previously not been reported in the literature. Due to the intravitreal air injection risk, it is important to be more careful while preparing the intravitreal medication before treatment in premature babies.

Similar content being viewed by others
References
Lad EM, Hernandez-Boussard T, Morton JM, Moshfeghi DM (2009) Incidence of retinopathy of prematurity in the United States: 1997 through 2005. Am J Ophthalmol 148(3):451–458
Cryotherapy for Retinopathy of Prematurity Cooperative Group (1990) Multicenter trial of cryotherapy for retinopathy of prematurity. One-year outcome—structure and function. Arch Ophthalmol 108(10):1408–1416
Early Treatment for Retinopathy of Prematurity Cooperative Group (2003) Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol 121(12):1684–1694
Mintz-Hittner HA, Kennedy KA, Chuang AZ, Beat-rop Cooperative Group (2011) Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med 364(7):603–615
Lai TY, Liu S, Das S, Lam DS (2015) Intravitreal injection-technique and safety. Asia Pac J Ophthalmol (Phila) 4:321–328
Kuniyoshi K, Sugioka K, Sakuramoto H, Kusaka S, Wada N, Shimomura Y (2014) Intravitreal injection of bevacizumab for retinopathy of prematurity. Jpn J Ophthalmol 58(3):237–243
Wu WC, Kuo HK, Yeh PT, Yang CM, Lai CC, Chen SN (2013) An updated study of the use of bevacizumab in the treatment of patients with prethreshold retinopathy of prematurity in taiwan. Am J Ophthalmol 155(1):150–158
Somner JE, Mansfield D (2009) In advertent injection of intravitreal air during intravitreal Lucentis injection for wet age-related macular degeneration: an un described complication. Eye 23(8):1744
Castellanos MA, Schwartz S, García-Aguirre G, Quiroz-Mercado H (2013) Short-term out come after intravitreal ranibizumab injections for the treatment of retinopathy of prematurity. Br J Ophthalmol 97(7):816–819
Hwang CK, Hubbard GB, Hutchinson AK, Lambert SR (2015) Outcomes after intravitreal bevacizumab versus laser photocoagulation for retinopathy of prematurity: a 5-year retrospective analysis. Ophthalmology 122(5):1008–1015
Yetik H, Gunay M, Sirop S, Salihoglu Z (2015) Intravitreal bevacizumab monotherapy for type-1 prethreshold, threshold, and aggressive posterior retinopathy of prematurity—27 month follow-up results from Turkey. Graefes Arch Clin Exp Ophthalmol 253(10):1677–1683
Shima C, Sakaguchi H, Gomi F, Kamei M, Ikuno Y, Oshima Y, Sawa M, Tsujikawa M, Kusaka S, Tano Y (2008) Complications in patients after intravitreal injection of bevacizumab. Acta Ophthalmol 86(4):372–376
Yun C, Oh J, Hwang SY, Kim SW, Huh K (2015) Subconjunctival hemorrhage after intravitreal injection of anti-vascular endothelial growth factor. Graefes Arch Clin Exp Ophthalmol 253(9):1465–1470
Erdogan G, Gunay BO, Unlu C, Gunay M, Ergin A (2015) Management of iatrogenic crystalline lens injury occurred during intravitreal injection. Int Ophthalmol: 1–4 [Epub ahead of print]
Weismann LE, Stoll BJ, Kueser TJ, Rubio TT, Frank CG, Heiman HS et al (1994) Intravenous immune globulin prophylaxis of late-onset sepsis in premature neonates. J Pediatr 125:922–930
Funding
The authors did not receive any financial support from any public or private sources. The authors have no financial or proprietary interest in a product, method or material described herein.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sukgen, E.A., Gunay, M. & Kocluk, Y. Occurrence of intraocular air bubbles during intravitreal injections for retinopathy of prematurity. Int Ophthalmol 37, 215–219 (2017). https://doi.org/10.1007/s10792-016-0257-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-016-0257-9