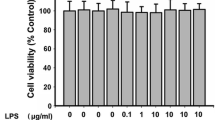
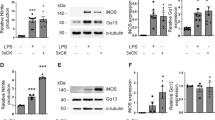
Abstract
Microglia activation has been implicated in the pathogenesis of many neurological diseases. These reactive microglia are capable of producing a variety of proinflammatory mediators and potentially neurotoxic compounds. The increase of cell number and expression of CD11b are the main features of activated microglia. In this study, we examined the suppressive effects of CDK11p58 on microglia activation induced by lipopolysaccharide (LPS) in vitro. We found that in the activated microglia, the expression of CDK11p58 increased and the overexpression of CDK11p58 could reduce the increased proliferation and CD11b expression in LPS-activated microglia. Such suppressive effects might be resulted from the interaction with cyclin D3 which promoted CDK11p58 nuclear localization. Our results suggested that CDK11p58 acted to regulate microglia activation through CDK11p58 and cyclin D3 interaction.





Similar content being viewed by others
Reference
Bajić, V.P., B. Su, H.G. Lee, W. Kudo, S.L. Siedlak, L. Zivković, B. Spremo-Potparević, N. Djelic, Z. Milicevic, A.K. Singh, L.M. Fahmy, X. Wang, M.A. Smith, and X. Zhu. 2011. Mislocalization of CDK11/PITSLRE, a regulator of the G2/M phase of the cell cycle, in Alzheimer disease. Cell Mol Biol Lett 16(3): 359–372. doi:10.2478/s11658-011-0011-2.
Behrendt, P., P. Arnold, M. Brueck, U. Rickert, R. Lucius, S. Hartmann, C. Klotz, and R. Lucius. 2016. A Helminth Protease Inhibitor Modulates the Lipopolysaccharide-Induced Proinflammatory Phenotype of Microglia in vitro. Neuroimmunomodulation 23(2): 109–121. doi:10.1159/000444756.
Drogat, J., V. Migeot, E. Mommaerts, C. Mullier, M. Dieu, H. van Bakel, and D. Hermand. 2012. Cdk11-cyclinL controls the assembly of the RNA polymerase II mediator complex. Cell Reports 2(5): 1068–1076. doi:10.1016/j.celrep.2012.09.027.
Erny, D., A.L. de Angelis, and M. Prinz. 2016. Communicating systems in the body: how microbiota and microglia cooperate. Immunology. doi:10.1111/imm.12645.
Fidler, I.J. 2015. The Biology of Brain Metastasis: Challenges for Therapy. Cancer Journal 21(4): 284–293. doi:10.1097/PPO.0000000000000126.
Gold, M., and J. El Khoury. 2015. Beta-amyloid, microglia, and the inflammasome in Alzheimer’s disease. Seminars in Immunopathology 37(6): 607–611. doi:10.1007/s00281-015-0518-0.
Goldmann, T., P. Wieghofer, P.F. Muller, Y. Wolf, D. Varol, S. Yona, S.M. Brendecke, K. Kierdorf, O. Staszewski, M. Datta, T. Luedde, M. Heikenwalder, S. Jung, and M. Prinz. 2013. A new type of microglia gene targeting shows TAK1 to be pivotal in CNS autoimmune inflammation. Nature Neuroscience 16(11): 1618–1626. doi:10.1038/nn.3531.
Guo, S., Y. Liu, R. Ma, J. Li, and B. Su. 2016. Neuroprotective effect of endogenous cannabinoids on ischemic brain injury induced by the excess microglia-mediated inflammation. American Journal of Translational Research 8(6): 2631–2640.
Hilton, G.D., B.A. Stoica, K.R. Byrnes, and A.I. Faden. 2008. Roscovitine reduces neuronal loss, glial activation, and neurologic deficits after brain trauma. Journal of Cerebral Blood flow and Metabolism : Official Journal of the International Society of Cerebral Blood Flow and Metabolism 28(11): 1845–1859. doi:10.1038/jcbfm.2008.75.
Joers, V., M.G. Tansey, G. Mulas, and A.R. Carta. 2016. Microglial phenotypes in Parkinson’s disease and animal models of the disease. Progress in Neurobiology. doi:10.1016/j.pneurobio.2016.04.006.
Lee, Y.Y., E.J. Lee, J.S. Park, S.E. Jang, D.H. Kim, and H.S. Kim. 2016. Anti-Inflammatory and Antioxidant Mechanism of Tangeretin in Activated Microglia. Journal of Neuroimmune Pharmacology : The Official Journal of the Society on NeuroImmune Pharmacology 11(2): 294–305. doi:10.1007/s11481-016-9657-x.
Lewis, D.K., A.B. Johnson, S. Stohlgren, A. Harms, and F. Sohrabji. 2008. Effects of estrogen receptor agonists on regulation of the inflammatory response in astrocytes from young adult and middle-aged female rats. Journal of Neuroimmunology 195(1–2): 47–59.
Martini, A.C., T. Berta, S. Forner, G. Chen, A.F. Bento, R.R. Ji, and G.A. Rae. 2016. Lipoxin A4 inhibits microglial activation and reduces neuroinflammation and neuropathic pain after spinal cord hemisection. Journal of Neuroinflammation 13(1): 75. doi:10.1186/s12974-016-0540-8.
Menet, V., M. Gimenez y Ribotta, N. Chauvet, M.J. Drian, J. Lannoy, E. Colucci-Guyon, and A. Privat. 2001. Inactivation of the glial fibrillary acidic protein gene, but not that of vimentin, improves neuronal survival and neurite growth by modifying adhesion molecule expression. The Journal of Neuroscience 21(16): 6147–6158.
Pastore N, Brady OA, Diab HI, Martina JA, Sun L, Huynh T, Lim JA, Zare H, Raben N, Ballabio A, Puertollano R. 2016. TFEB and TFE3 cooperate in the regulation of the innate immune response in activated macrophages. Autophagy 1–19. doi:10.1080/15548627.2016.1179405
Petretti, C., M. Savoian, E. Montembault, D.M. Glover, C. Prigent, and R. Giet. 2006. The PITSLRE/CDK11p58 protein kinase promotes centrosome maturation and bipolar spindle formation. EMBO Reports 7(4): 418–424. doi:10.1038/sj.embor.7400639.
Tateda, S., H. Kanno, H. Ozawa, A. Sekiguchi, K. Yahata, S. Yamaya, and E. Itoi. 2016. Rapamycin suppresses microglial activation and reduces the development of neuropathic pain after spinal cord injury. Journal of Orthopaedic Research : Official Publication of the Orthopaedic Research Society. doi:10.1002/jor.23328.
Tsou, Y.C., H.H. Wang, C.C. Hsieh, K.H. Sun, G.H. Sun, R.S. Jhou, T.I. Lin, S.Y. Lu, H.Y. Liu, and S.J. Tang. 2016. Down-regulation of BNIP3 by olomoucine, a CDK inhibitor, reduces LPS- and NO-induced cell death in BV2 microglial cells. Neuroscience Letters 628: 186–193. doi:10.1016/j.neulet.2016.06.040.
Yuan, Y., M. Fang, C.Y. Wu, and E.A. Ling. 2016. Scutellarin as a Potential Therapeutic Agent for Microglia-Mediated Neuroinflammation in Cerebral Ischemia. Neuromolecular Medicine. doi:10.1007/s12017-016-8394-x.
Zhang, C., M. Zhang, Q. Wu, J. Peng, Y. Ruan, and J. Gu. 2015. Hepsin inhibits CDK11p58 I.E. activity by suppressing unr expression and eIF-2α phosphorylation in prostate cancer. Cellular Signalling 27(4): 789–797. doi:10.1016/j.cellsig.2014.12.020.
Zhang, S., M. Cai, S. Zhang, S. Xu, S. Chen, X. Chen, C. Chen, and J. Gu. 2002. Interaction of p58(PITSLRE), a G2/M-specific protein kinase, with cyclin D3. The Journal of Biological Chemistry 277(38): 35314–35322.
Zhou, Y., Y.J. Guo, X. Liu, and Y.W. Mei. 2009. Cell cycle inhibitor enhances the resolution of HSV-1-induced proinflammatory response in murine microglial cells. Neurological Research 31(9): 910–916. doi:10.1179/174313209X383222.
Zhou, Y., J.K. Shen, F.J. Hornicek, Q. Kan, and Z. Duan. 2016. The emerging roles and therapeutic potential of cyclin-dependent kinase 11 (CDK11) in human cancer. Oncotarget. doi:10.18632/oncotarget.8519.
Zong, H., Y. Chi, Y. Wang, Y. Yang, L. Zhang, H. Chen, J. Jiang, Z. Li, Y. Hong, H. Wang, X. Yun, and J. Gu. 2007. Cyclin D3/CDK11p58 complex is involved in the repression of androgen receptor. Molecular and Cellular Biology 27(20): 7125–7142. doi:10.1128/MCB.01753-06.
Acknowledgments
This work was supported by grants from Chinese National Natural Science Foundation (Nos 81401124), Social Science and Technology Innovation and Demonstration Foundation of Nantong City (MS22015003); Preventive Medicine Projects from Bureau of Jiangsu Province (Y2012083);“Top Six Types of Talents” Financial Assistance of Jiangsu Province (Grant no. 10.WSN016).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Biyu Shen and Tianyu Gu contributed equally to this work
Rights and permissions
About this article
Cite this article
Shen, B., Gu, T., Chen, H. et al. CDK11p58 Promotes Microglia Activation via Inducing Cyclin D3 Nuclear Localization. Inflammation 40, 636–644 (2017). https://doi.org/10.1007/s10753-017-0510-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-017-0510-z