Abstract
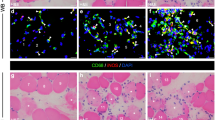
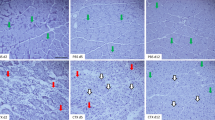
Muscle contusion is one of the most common muscle injuries in sports medicine. Macrophages play complex roles in the regeneration of skeletal muscle. However, the roles of macrophages, especially the mechanisms involved, in the regeneration of muscle contusion are still not fully understood. We hypothesize that the depletion of macrophages impairs skeletal muscle regeneration and that pro-fibrotic factors, inflammation, and oxidative stress may be involved in the process. To test these hypotheses, we constructed a muscle contusion injury and a macrophage depletion model and followed it up with morphological and gene expression analyses. The data showed that fibrotic scars were formed in the muscle of contusion injury, and they deteriorated in the mice of macrophage depletion. Furthermore, the sizes of regenerating myofibers were significantly reduced by macrophage depletion. Pro-fibrotic factors, inflammatory cytokines, chemokines, and oxidative stress-related enzymes increased significantly after muscle injury. Moreover, the expression of these factors was delayed by macrophage depletion. Most of them were still significantly higher in the later stage of regeneration. These results suggest that macrophage depletion impairs skeletal muscle regeneration and that pro-fibrotic factors, inflammation, and oxidative stress may play important roles in the process.







Similar content being viewed by others
Abbreviations
- GM:
-
Gastrocnemius muscle
- CL-liposomes:
-
Clodronate-containing (CL) liposomes
- HE staining:
-
Hematoxylin and eosin staining
- PCR:
-
Polymerase chain reaction
- TGF-beta 1:
-
Transforming growth factor-beta 1
- TNF-alpha:
-
Tumor necrosis factor (TNF)-alpha
- IL-1 beta:
-
Interleukin (IL)-1beta
- IL-6:
-
Interleukin (IL)-6
- IL-10:
-
Interleukin (IL)-10
- CCL2:
-
CC ligand-2
- CXCL10:
-
CXC ligand-10
- NADPH-oxidases:
-
Nicotinamide adenine dinucleotide phosphate (NADPH)-oxidases
- ROS:
-
Reactive oxygen species
References
Nozaki, M., Y. Li, J. Zhu, F. Ambrosio, K. Uehara, F.H. Fu, and J. Huard. 2008. Improved muscle healing after contusion injury by the inhibitory effect of suramin on myostatin, a negative regulator of muscle growth. The American Journal of Sports Medicine 36(12): 2354–2362.
Tidball, J.G., and S.A. Villalta. 2010. Regulatory interactions between muscle and the immune system during muscle regeneration. American Journal of Physiology—Regulatory, Integrative and Comparative Physiology 298(5): R1173–R1187.
Honda, H., H. Kimura, and A. Rostami. 1990. Demonstration and phenotypic characterization of resident macrophages in rat skeletal muscle. Immunology 70(2): 272–277.
Hurme, T., H. Kalimo, M. Lehto, and M. Jarvinen. 1991. Healing of skeletal muscle injury: an ultrastructural and immunohistochemical study. Medicine and Science in Sports and Exercise 23(7): 801–810.
Carlson, B.M., and J.A. Faulkner. 1983. The regeneration of skeletal muscle fibers following injury: a review. Medicine and Science in Sports and Exercise 15(3): 187–198.
Summan, M., G.L. Warren, R.R. Mercer, R. Chapman, T. Hulderman, N. Van Rooijen, and P.P. Simeonova. 2006. Macrophages and skeletal muscle regeneration: a clodronate-containing liposome depletion study. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 290(6): R1488–R1495.
Bosurgi, L., A.A. Manfredi, and P. Rovere-Querini. 2011. Macrophages in injured skeletal muscle: a perpetuum mobile causing and limiting fibrosis, prompting or restricting resolution and regeneration. Frontiers in Immunology 2: 62.
Kasemkijwattana, C., J. Menetrey, G. Somogyi, M.S. Moreland, F.H. Fu, B. Buranapanitkit, S.C. Watkins, and J. Huard. 1998. Development of approaches to improve the healing following muscle contusion. Cell Transplantation 7(6): 585–598.
Wright-Carpenter, T., P. Opolon, H. Appell, H. Meijer, P. Wehling, and L. Mir. 2004. Treatment of muscle injuries by local administration of autologous conditioned serum: animal experiments using a muscle contusion model. International Journal of Sports Medicine 25(8): 582–587.
Xiao, W.H., Y. Liu, B.B. Luo, L.L. Zhao, X.G. Liu, Z.G. Zeng, and P.J. Chen. 2016. Time-dependent gene expression analysis after mouse skeletal muscle contusion. Journal of Sport and Health Science 5(1): 101–108.
Fisher, B.D., V.E. Baracos, T.K. Shnitka, S.W. Mendryk, and D.C. Reid. 1990. Ultrastructural events following acute muscle trauma. Medicine and Science in Sports and Exercise 22(2): 185–193.
Crisco, J.J., P. Jokl, G.T. Heinen, M.D. Connell, and M.M. Panjabi. 1994. A muscle contusion injury model biomechanics, physiology, and histology. The American Journal of Sports Medicine 22(5): 702–710.
Diaz, J.A., D.A. Fischer, A.C. Rettig, T.J. Davis, and K.D. Shelbourne. 2003. Severe quadriceps muscle contusions in athletes. A report of three cases. The American Journal of Sports Medicine 31(2): 289–293.
Dobek, G.L., N.D. Fulkerson, J. Nicholas, and B.S. Schneider. 2013. Mouse model of muscle crush injury of the legs. Comparative Medicine 63(3): 227–232.
Tyner, J.W., O. Uchida, N. Kajiwara, E.Y. Kim, A.C. Patel, M.P. O’Sullivan, M.J. Walter, R.A. Schwendener, D.N. Cook, T.M. Danoff, et al. 2005. CCL5-CCR5 interaction provides antiapoptotic signals for macrophage survival during viral infection. Nature Medicine 11(11): 1180–1187.
Popovich, P.G., Z. Guan, P. Wei, I. Huitinga, N. van Rooijen, and B.T. Stokes. 1999. Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Experimental Neurology 158(2): 351–365.
Bacci, M., A. Capobianco, A. Monno, L. Cottone, F. Di Puppo, B. Camisa, M. Mariani, C. Brignole, M. Ponzoni, S. Ferrari, et al. 2009. Macrophages are alternatively activated in patients with endometriosis and required for growth and vascularization of lesions in a mouse model of disease. The American Journal of Pathology 175(2): 547–556.
Holt, M.P., L. Cheng, and C. Ju. 2008. Identification and characterization of infiltrating macrophages in acetaminophen-induced liver injury. Journal of Leukocyte Biology 84(6): 1410–1421.
Shen, W., Y. Li, J. Zhu, R. Schwendener, and J. Huard. 2008. Interaction between macrophages, TGF-beta1, and the COX-2 pathway during the inflammatory phase of skeletal muscle healing after injury. Journal of Cellular Physiology 214(2): 405–412.
Foster, W., Y. Li, A. Usas, G. Somogyi, and J. Huard. 2003. Gamma interferon as an antifibrosis agent in skeletal muscle. Journal of orthopaedic research : official publication of the Orthopaedic Research Society 21(5): 798–804.
Fukushima, K., N. Badlani, A. Usas, F. Riano, F. Fu, and J. Huard. 2001. The use of an antifibrosis agent to improve muscle recovery after laceration. The American Journal of Sports Medicine 29(4): 394–402.
Chirgwin, J.M., A.E. Przybyla, R.J. MacDonald, and W.J. Rutter. 1979. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry 18(24): 5294–5299.
Livak, K.J., and T.D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25(4): 402–408.
Villalta, S.A., C. Rinaldi, B. Deng, G. Liu, B. Fedor, and J.G. Tidball. 2011. Interleukin-10 reduces the pathology of mdx muscular dystrophy by deactivating M1 macrophages and modulating macrophage phenotype. Human Molecular Genetics 20(4): 790–805.
Mann, C.J., E. Perdiguero, Y. Kharraz, S. Aguilar, P. Pessina, A.L. Serrano, and P. Munoz-Canoves. 2011. Aberrant repair and fibrosis development in skeletal muscle. Skeletal Muscle 1(1): 21.
Li, Z.B., H.D. Kollias, and K.R. Wagner. 2008. Myostatin directly regulates skeletal muscle fibrosis. The Journal of Biological Chemistry 283(28): 19371–19378.
Chan, E.C., F. Jiang, H.M. Peshavariya, and G.J. Dusting. 2009. Regulation of cell proliferation by NADPH oxidase-mediated signaling: potential roles in tissue repair, regenerative medicine and tissue engineering. Pharmacology & Therapeutics 122(2): 97–108.
Cabello-Verrugio, C., M.J. Acuna, M.G. Morales, A. Becerra, F. Simon, and E. Brandan. 2011. Fibrotic response induced by angiotensin-II requires NAD(P)H oxidase-induced reactive oxygen species (ROS) in skeletal muscle cells. Biochemical and Biophysical Research Communications 410(3): 665–670.
Nishimura, S., I. Manabe, M. Nagasaki, K. Eto, H. Yamashita, M. Ohsugi, M. Otsu, K. Hara, K. Ueki, S. Sugiura, et al. 2009. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nature Medicine 15(8): 914–920.
Stoneman, V., D. Braganza, N. Figg, J. Mercer, R. Lang, M. Goddard, and M. Bennett. 2007. Monocyte/macrophage suppression in CD11b diphtheria toxin receptor transgenic mice differentially affects atherogenesis and established plaques. Circulation Research 100(6): 884–893.
Xiao, W., P. Chen, and J. Dong. 2012. Effects of overtraining on skeletal muscle growth and gene expression. International Journal of Sports Medicine 33(10): 846–853.
Chan, Y.S., Y. Li, W. Foster, T. Horaguchi, G. Somogyi, F.H. Fu, and J. Huard. 2003. Antifibrotic effects of suramin in injured skeletal muscle after laceration. Journal of Applied Physiology 95(2): 771–780.
Segawa, M., S. Fukada, Y. Yamamoto, H. Yahagi, M. Kanematsu, M. Sato, T. Ito, A. Uezumi, S. Hayashi, Y. Miyagoe-Suzuki, et al. 2008. Suppression of macrophage functions impairs skeletal muscle regeneration with severe fibrosis. Experimental Cell Research 314(17): 3232–3244.
Uezumi, A., T. Ito, D. Morikawa, N. Shimizu, T. Yoneda, M. Segawa, M. Yamaguchi, R. Ogawa, M.M. Matev, Y. Miyagoe-Suzuki, et al. 2011. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. Journal of Cell Science 124(Pt 21): 3654–3664.
Li, Y., W. Foster, B.M. Deasy, Y. Chan, V. Prisk, Y. Tang, J. Cummins, and J. Huard. 2004. Transforming growth factor-beta1 induces the differentiation of myogenic cells into fibrotic cells in injured skeletal muscle: a key event in muscle fibrogenesis. The American Journal of Pathology 164(3): 1007–1019.
Li, Y., and J. Huard. 2002. Differentiation of muscle-derived cells into myofibroblasts in injured skeletal muscle. The American Journal of Pathology 161(3): 895–907.
Zhu, J., Y. Li, W. Shen, C. Qiao, F. Ambrosio, M. Lavasani, M. Nozaki, M.F. Branca, and J. Huard. 2007. Relationships between transforming growth factor-beta1, myostatin, and decorin: implications for skeletal muscle fibrosis. The Journal of Biological Chemistry 282(35): 25852–25863.
Urso, M.L. 2013. Anti-inflammatory interventions and skeletal muscle injury: benefit or detriment? Journal of Applied Physiology 115(6): 920–928.
Meyer, G.A., and R.L. Lieber. 2012. Skeletal muscle fibrosis develops in response to desmin deletion. American Journal of Physiology. Cell Physiology 302(11): C1609–C1620.
Ermolova, N.V., L. Martinez, S.A. Vetrone, M.C. Jordan, K.P. Roos, H.L. Sweeney, and M.J. Spencer. 2014. Long-term administration of the TNF blocking drug Remicade (cV1q) to mdx mice reduces skeletal and cardiac muscle fibrosis, but negatively impacts cardiac function. Neuromuscular Disorders 24(7): 583–595.
Abdelmagid, S.M., A.E. Barr, M. Rico, M. Amin, J. Litvin, S.N. Popoff, F.F. Safadi, and M.F. Barbe. 2012. Performance of repetitive tasks induces decreased grip strength and increased fibrogenic proteins in skeletal muscle: role of force and inflammation. PLoS One 7(5): e38359.
Ghaly, A., and D.R. Marsh. 2010. Ischaemia-reperfusion modulates inflammation and fibrosis of skeletal muscle after contusion injury. International Journal of Experimental Pathology 91(3): 244–255.
Xiao, W., P. Chen, R. Wang, and J. Dong. 2013. Overload training inhibits phagocytosis and ROS generation of peritoneal macrophages: role of IGF-1 and MGF. European Journal of Applied Physiology 113(1): 117–125.
Xiao, W., P. Chen, J. Dong, R. Wang, and B. Luo. 2014. Dietary Glutamine Supplementation Partly Reverses Impaired Macrophage Function Resulting From Overload Training in Rats. International Journal of Sport Nutrition and Exercise 25(2): 179–187.
Wynn, T.A., and L. Barron. 2010. Macrophages: master regulators of inflammation and fibrosis. Seminars in Liver Disease 30(3): 245–257.
Long, K.K., M. Montano, and G.K. Pavlath. 2011. Sca-1 is negatively regulated by TGF-beta1 in myogenic cells. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 25(4): 1156–1165.
Acknowledgments
This work was supported by the grants from the National Natural Science Foundation of China (31271273, 31300975), the Doctoral Fund of Ministry of Education of China (20133156120004) and the Key Lab of Exercise and Health Sciences of Ministry of Education (Shanghai University of Sport).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Authors’ Contributions
WX and PC analyzed and interpreted the data. YL performed the histological examination of the skeletal muscle. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Xiao, W., Liu, Y. & Chen, P. Macrophage Depletion Impairs Skeletal Muscle Regeneration: the Roles of Pro-fibrotic Factors, Inflammation, and Oxidative Stress. Inflammation 39, 2016–2028 (2016). https://doi.org/10.1007/s10753-016-0438-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-016-0438-8