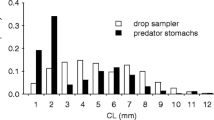
Abstract
This study assessed effects of abiotic (current velocity, water depth, particle size) and biotic (predation risk for crayfish, size distribution and densities of predatory fish) variables on habitat- and size-specific distribution patterns of lotic crayfish (Orconectes obscurus) using field surveys and tethering experiments. Additionally, particle size manipulations were used with predation assays to assess habitat-specific interactions since the average particle size increased from deep pools to shallow pools to riffles. Large crayfish had the highest densities in deep pools and were associated with increased water depth, whereas small and medium crayfish had the highest densities in shallow pools and were strongly associated with increased particle size and decreased water depth. Regardless of size, crayfish in deep pools had significantly lower survival than in shallow pools and riffles. However, only small crayfish showed consistent differences in predation risk by habitat type and were significantly more vulnerable to predation than larger crayfish. Additionally, large rocky refugia resulted in significantly higher survival of small crayfish in the combined particle manipulation/tethering experiment. Overall, predation appears to be a key mechanism structuring habitat-specific distribution patterns for only small O. obscurus. Large substrates may be particularly important in habitats where both small crayfish density and predation risk are high.



Similar content being viewed by others
References
Bovbjerg, R. V., 1956. Some factors affecting aggressive behavior in crayfish. Physiological Zoology 29: 127–136.
Bubb, D. H., T. J. Thom & M. C. Lucas, 2004. Movement and dispersal of the invasive signal crayfish Pacifastacus leniusculus in upland rivers. Freshwater Biology 49: 357–368.
Clark, J. M., M. W. Kershner & J. R. Holomuzki, 2008. Grain size and sorting effects on size-dependent responses by lotic crayfish to high flows. Hydrobiologia 610: 55–66.
Correia, A. M., 2001. Seasonal and interspecific evaluation of predation by mammals and birds on the introduced red swamp crayfish Procambarus clarkii (Crustacea, Cambaridae) in a freshwater marsh (Portugal). Journal of Zoology 255: 533–541.
Cox, D. R., 1972. Regression models and life tables. Journal of the Royal Statistical Society 34: 187–200.
Creed, R. P., 2006. Predator transitions in stream communities: a model and evidence from field studies. Journal of the North American Benthological Society 25: 533–544.
Creed, R. P. & J. M. Reed, 2004. Ecosystem engineering by crayfish in a headwater stream community. Journal of the North American Benthological Society 23: 224–236.
DiDonato, G. T. & D. M. Lodge, 1993. Species replacements among Orconectes crayfishes in Wisconsin Lakes-The role of predation by fish. Canadian Journal of Fisheries and Aquatic Sciences 50: 1484–1488.
Dorn, N. J. & G. G. Mittelbach, 1999. More than predator and prey: a review of interactions between fish and crayfish. Vie et Milieu-Life and Environment 49: 229–327.
Englund, G. & J. J. Krupa, 2000. Habitat use by crayfish in stream pools: influence of predators, depth, and body size. Freshwater Biology 43: 75–83.
Fielder, D. D., 1972. Some aspects of the life histories of three closely related crayfish species, Orconectes obscurus, O. sanborni, and O. propinquus. The Ohio Journal of Science 72: 129–145.
Flinders, C. A. & D. D. Magoulick, 2003. Effects of stream permanence on crayfish community structure. American Midland Naturalist 149: 134–147.
Flinders, C. A. & D. D. Magoulick, 2005. Distribution, habitat use and life history of stream- dwelling crayfish in the spring river drainage of Arkansas and Missouri with a focus on the imperiled Mammoth Spring crayfish (Orconectes marchandi). American Midland Naturalist 154: 358–374.
Flinders, C. A. & D. D. Magoulick, 2007a. Effects of depth and crayfish size on predation risk and foraging profitability of a lotic crayfish. Journal of the North American Benthological Society 26: 767–778.
Flinders, C. A. & D. D. Magoulick, 2007b. Habitat use and selection within Ozark lotic crayfish assemblages: spatial and temporal variation. Journal of Crustacean Biology 27: 242–254.
Garvey, J. E., R. A. Stein & H. M. Thomas, 1994. Assessing how fish predation and interspecific prey competition influence a crayfish assemblage. Ecology 75: 532–547.
Gordon, N. D., T. A. McMahon, B. L. Finlayson, C. J. Gippel & R. J. Nathan, 2004. Stream Hydrology: An Introduction for Ecologists, 2nd ed. Wiley, West Sussex, England.
Griffith, M. B., S. A. Perry & W. B. Perry, 1994. Secondary production of macroinvertebrate shredders in headwater streams with different baseflow alkalinity. Journal of the North American Benthological Society 13: 345–356.
Hart, D. D. & C. M. Finelli, 1999. Physical-biological coupling in streams: the pervasive effects of flow on benthic organisms. Annual Review of Ecology and Systematics 30: 363–395.
Hicks, B. J., 2003. Distribution of fish and crayfish in a Waikato stream in relation to basin area. New Zealand Journal of Zoology 30: 149–160.
Hill, A. M. & D. M. Lodge, 1999. Replacement of resident crayfishes by an exotic crayfish: the roles of competition and predation. Ecological Applications 9: 678–690.
Kershner, M. W. & D. M. Lodge, 1995. Effects of littoral habitat and fish predation on the distribution of an exotic crayfish, Orconectes rusticus. Journal of the North American Benthological Society 14: 414–422.
Kuhlmann, M. L., S. M. Badylak & E. L. Carvin, 2008. Testing the differential predation hypothesis for the invasion of rusty crayfish in a stream community: laboratory and field experiments. Freshwater Biology 53: 113–128.
Legendre, P. & L. Legendre, 1998. Numerical Ecology. Elsevier Science, Amsterdam, The Netherlands.
Light, T., 2003. Success and failure in a lotic crayfish invasion: the roles of hydrologic variability and habitat alteration. Freshwater Biology 48: 1886–1897.
Lodge, D. M. & A. M. Hill, 1994. Factors governing species composition, population size, and productivity of cool-water crayfishes. Nordic Journal of Freshwater Research 69: 111–136.
Lodge, D. M., M. W. Kershner, J. E. Aloi & A. P. Covich, 1994. Effects of omnivorous crayfish (Orconectes rusticus) on a freshwater food web. Ecology. 75: 265–1281.
Lodge, D. M., C. A. Taylor, D. M. Holdich & J. Skurdal, 2000. Nonindigenous crayfishes threaten North American freshwater biodiversity: lessons from Europe. Fisheries 25: 7–20.
Magoulick, D. D., 2004. Effects of predation risk on habitat selection by water column fish, benthic fish, and crayfish in stream pools. Hydrobiologia 527: 209–221.
Mather, M. E. & R. A. Stein, 1993a. Direct and indirect effects of fish predation on the replacement of a native crayfish by an invading congener. Canadian Journal of Fisheries and Aquatic Sciences 50: 1279–1288.
Mather, M. E. & R. A. Stein, 1993b. Using growth-mortality tradeoffs to explore a crayfish species replacement in stream riffles and pools. Canadian Journal of Fisheries and Aquatic Sciences 50: 88–96.
McCune, B., J. B. Grace & D. L. Urban, 2002. Analysis of Ecological Communities. MjM Software Design, Gleneden Beach, OR.
Nyström, P., C. Brönmark & W. Granéli, 1996. Patterns in benthic food webs: a role for omnivorous crayfish? Freshwater Biology 36: 631–646.
Oksanen, J., F. G. Blanchet, R Kindt, P. Legendre, P. R. Minchin, R. B. O’Hara, G. L. Simpson, P. Solymos, M. Henry, H. Stevens & H. Wagner, 2013. vegan: Community Ecology Package. R package version 2.0-6. http://CRAN.R-project.org/package=vegan/.
Olsson, K. & P. Nyström, 2009. Non-interactive effects of habitat complexity and adult crayfish on survival and growth of juvenile crayfish (Pacifastacus leniusculus). Freshwater Biology 54: 35–46.
Parkyn, S. M. & K. J. Collier, 2004. Interaction of press and pulse disturbance on crayfish populations: flood impacts in pasture and forest streams. Hydrobiologia 527: 114–124.
Pockl, M. & F. Streissl, 2005. Austropotamobius torrentium as an indicator for habitat quality in running waters? Bulletin Francais De La Peche Et De La Pisciculture 376–377: 743–758.
Poos, M. S., N. E. Mandrak & R. L. McLaughlin, 2007. The effectiveness of two common sampling methods for assessing imperiled freshwater fishes. Journal of Fish Biology 70: 691–708.
R Core Team, 2013. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/.
Rabeni, C. F., 1992. Trophic linkage between stream Centrarchids and their crayfish prey. Canadian Journal of Fisheries and Aquatic Sciences 49: 1714–1721.
Rabeni, C. F., K. J. Collier, S. M. Parkyn & B. J. Hicks, 1997. Evaluating techniques for sampling stream crayfish (Paranephrops planifrons). New Zealand Journal of Marine and Freshwater Research 31: 693–700.
Renai, B. & F. Gherardi, 2004. Predatory efficiency of crayfish: comparison between indigenous and non-indigenous species. Biological Invasions 6: 89–99.
Roell, M. J. & D. J. Orth, 1993. Trophic basis of stream-dwelling smallmouth bass, rock bass, and flathead catfish in relation to invertebrate bait harvest. Transactions of the American Fisheries Society 122: 46–62.
SAS Institute, 2000. The SAS System for Windows, version 8.01. SAS Institute, Inc., Cary, North Carolina.
SAS Institute, 2007. JMP, version 7.0.1. SAS Institute, Inc., Cary, North Carolina.
Schlosser, I. J., 1987. The role of predation in age- and size-related habitat use by stream fishes. Ecology 68: 651–659.
Seiler, S. M. & A. M. Turner, 2004. Growth and population size of crayfish in headwater streams: individual- and higher-level consequences of acidification. Freshwater Biology 49: 870–881.
Statzner, B., O. Peltret & S. Tomanova, 2003. Crayfish a geomorphic agents and ecosystem Engineers: effect of a biomass gradient on baseflow and flood-induced transport of gravel and sand in experimental streams. Freshwater Biology 48: 147–163.
Stein, R. A., 1977. Selective predation, optimal foraging, and the predator-prey interaction between fish and crayfish. Ecology 58: 1237–1253.
Stein, R. A. & J. J. Magnuson, 1976. Behavioral response of a crayfish to a fish predator. Ecology 57: 751–761.
Streissl, F. & W. Hödl, 2002. Habitat and shelter requirements of the stone crayfish, Austropotamobius torrentium Schrank. Hydrobiologia 477: 195–199.
Trautman, M. B., 1957. Fishes of Ohio. The Ohio State University Press, Ohio.
Usio, N. & C. R. Townsend, 2000. Distribution of the New Zealand crayfish Paranephrops zealandicus in relation to stream physico-chemistry, predatory fish, and invertebrate prey. New Zealand Journal of Marine and Freshwater Research 34: 557–567.
Usio, N. & C. R. Townsend, 2001. The significance of the crayfish Paranephrops zealandicus as shredders in a New Zealand headwater stream. Journal of Crustacean Biology 21: 354–359.
Werner, E. E., J. F. Gilliam, D. J. Hall & G. G. Mittelbach, 1983a. Experimental tests of optimal habitat use in fish: the role of relative habitat profitability. Ecology 64: 1525–1539.
Werner, E. E., J. F. Gilliam, D. J. Hall & G. G. Mittelbach, 1983b. An experimental test of the effects of predation risk on habitat use in fish. Ecology 64: 1540–1548.
Wolman, M. G., 1954. A method of sampling coarse river-bed material. Transactions of the American Geophysical Union 15: 951–956.
Zippin, C., 1958. The removal method of population estimation. Journal of Wildlife Management 22: 82–90.
Acknowledgments
We would like to thank Matt Begley, Doug Kapusinski, Emily Faulkner, Joe Faulkner, Eric Floro, Emma Kennedy, Jackie Johnston, Joel Mulder, Jamie Stamberger, Matt Walker, Raja Vukanti, Maureen Drinkard, Adam Leff, Ben Leff, Laura Leff, Denise Walker, Constance Hausman, Stephanie Hovatter, Alex Arp, and Mark DuFour for assisting with fieldwork. Andrew Moore provided assistance with analysis of particle populations. Special thanks to two anonymous reviewers for providing helpful comments for an earlier version of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Lee B. Kats
Rights and permissions
About this article
Cite this article
Clark, J.M., Kershner, M.W. & Montemarano, J.J. Habitat-specific effects of particle size, current velocity, water depth, and predation risk on size-dependent crayfish distribution. Hydrobiologia 716, 103–114 (2013). https://doi.org/10.1007/s10750-013-1548-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-013-1548-z