Abstract
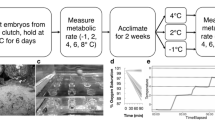
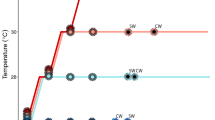
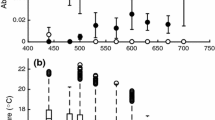
Juvenile ribbontail stingrays, Taeniura lymma (Forsskål, 1775) of the tropical West Pacific inhabit mangal and seagrass nurseries that often experience rapid and extreme increases in water temperature. We hypothesized that juvenile rays possess a thermal strategy similar to other hyperthermic specialists, in which fish prefer high temperatures, are always prepared for thermal extremes regardless of previous thermal history, and exhibit low metabolic thermal sensitivity. Critical thermal methodology was used to determine the thermal niche, and a thermal gradient used to estimate stingray final preferendum. Temperature quotients (Q 10) were calculated from metabolic rates determined at three temperatures using flow-through respirometry. As predicted, juvenile rays showed a relatively small thermal niche dominated by intrinsic tolerance with limited capacity for acclimation. Thermal preference values were higher than those reported for other elasmobranch species. Interestingly, the temperature quotient for juvenile rays was higher than expected, suggesting that these fish may have the ability to exploit the thermal heterogeneity in their environment. Temperature likely acts as a directing factor in this species, separating warm tolerant juveniles from adults living in deeper, cooler waters.


Similar content being viewed by others
References
Angilletta, M. J. Jr., A. F. Bennett, H. Guderley, C. A. Navas, F. Seebacher & R. S. Wilson, 2006. Coadaptation: a unifying principle in evolutionary thermal biology. Physiological and Biochemical Zoology 79: 282–294.
Baldwin, C. M., D. A. Beauchamp & C. P. Gubala, 2002. Seasonal and diel distribution and movement of cutthroat trout from ultrasonic telemetry. Transactions of the American Fisheries Society 131: 143–158.
Becker, C. D. & R. G. Genoway, 1979. Evaluation of the critical thermal maximum for determining thermal tolerance of freshwater fish. Environmental Biology of Fishes 4: 245–256.
Beitinger, T. L. & R. W. McCauley, 1990. Whole-animal physiological processes for the assessment of stress in fishes. International Association for Great Lakes Research 16: 542–575.
Beitinger, T. L., W. A. Bennett & R. W. McCauley, 2000. Temperature tolerances of North American freshwater fishes exposed to dynamic changes in temperature. Environmental Biology of Fishes 58: 237–275.
Bennett, W. A., 2010. Extreme physiology of intertidal fishes of the Wakatobi. In Clifton, J., R. K. F. Unsworth & D. J. Smith (eds), Marine research and conservation in the Coral Triangle: The Wakatobi National Park. Nova Science Publishers, Hauppauge, NY.
Bennett, W. A. & T. L. Beitinger, 1997. Temperature tolerance of the sheepshead minnow, Cyprinodon variegatus. Copeia 1997: 77–87.
Bennett, W. A., R. W. McCauley & T. L. Beitinger, 1998. Rates of gain and loss of heat tolerance in channel catfish. Transactions of the American Fisheries Society 127: 1053–1060.
Berge, H. B, 2009. Effects of a temperature-oxygen squeeze on distribution, feeding, growth, and survival of kokanee (Oncorhynchus nerka) in Lake Sammamish, Washington, Dissertation, University of Washington, Seattle.
Carlson, J. K. & G. R. Parsons, 1999. Seasonal differences in routine oxygen consumption rates of the bonnethead shark. Journal of Fish Biology 55: 876–879.
Casterlin, M. E. & W. W. Reynolds, 1979. Shark thermoregulation. Comparative Biochemistry and Physiology 64A: 451–453.
Cavanagh, R. D., P. M. Kyne, S. L. Fowler, J. A. Musick & M. B. Bennett (eds), 2003. The Conservation Status of Australian Chondrichthyans: Report of the IUCN Shark Specialist Group Australia and Oceania Regional Red List Workshop. University of Queensland, School of Biomedical Sciences, Brisbane.
Cech, J. J., 1990. Respirometry. In Schreck, C. B. & P. B. Moyle (eds), Methods for Fish Biology. American Fisheries Society, Bethesda: 335–356.
Chin, A., M. K. Peter, T. I. Walker & R. B. McAuley, 2010. An integrated risk assessment for climate change: analyzing the vulnerability of sharks and rays on Australia’s Great Barrier Reef Global Change Biology 16: 1936–1953.
Chung, K. S., 1980. Rate of acclimation of the tropical saltmarsh fish Cyprinodon dearborni to temperature changes. Hydrobiologia 78: 177–181.
Claussen, D. L., 1977. Thermal acclimation in ambystomatid salamanders. Comparative Biochemistry and Physiology 58A: 333–340.
Coutant, C. C., 1985. Striped bass, temperature, and dissolved oxygen: a speculative hypothesis for environmental risk. Transactions of the American Fisheries Society 114: 31–61.
Coutant, C. C., 1977. Compilation of temperature preference data. Journal of the Fisheries Research Board of Canada 34: 739–745.
Cowles, R. B. & C. M. Bogert, 1944. A preliminary study of the thermal requirements of desert reptiles. Bulletin of the American Museum of Natural History 83: 265–296.
Cox, D. K., 1974. Effects of three heating rates on the critical thermal maximum of bluegill. In Gibbons, J. W., & R. R. Sharitz (eds), Thermal Ecology Conference No. 730505. National Technical Information Service, Springfield, VA: 158–163.
Cox, G. W., 1990. Laboratory Manual of General Ecology, 6th ed. William C. Brown Publishers, Dubuque.
Crawshaw, L. I. & H. T. Hammel, 1973. Behavioral temperature regulation in the California horn shark, Heterodontus francisci. Brain, Behavior and Evolution 7: 447–452.
DiGirolamo, A. L., S. H. Gruber, C. Pomory & W. A. Bennett, 2012. Diel patterns of temperature selection from juvenile lemon sharks, Negaprion brevirostris, in a shallow water nursery. Journal of Fish Biology 80: 1436–1448.
Di Santo, V. & W. A. Bennett, 2011. Is post-feeding thermotaxis advantageous in elasmobranch fishes? Journal of Fish Biology 78: 195–207.
El-dawi, E. F. A., 2000. Diversity, habitats & seasonal distributions of fish in three protectorates of Sinai on the Red Sea. Egypt. Qatar University Science Journal 20: 111–124.
Eme, J. & W. A. Bennett, 2009a. Acute temperature quotient responses of fishes reflect their divergent thermal habitats in the Banda Sea, Sulawesi, Indonesia. Australian Journal of Zoology 57: 357–362.
Eme, J. & W. A. Bennett, 2009b. Critical thermal tolerance polygons of tropical marine fishes from Sulawesi, Indonesia. Journal of Thermal Biology 34: 220–225.
Fangue, N. A. & W. A. Bennett, 2003. Thermal tolerance responses of laboratory-acclimated and seasonally-acclimatized Atlantic stingray, Dasyatis sabina. Copeia 2003: 315–325.
Fast, A. W., 1973. Effects of artificial hypolimnion aeration on rainbow trout (Salmo gairdneri) depth distributions in a northern Michigan lake. Transactions of the American Fisheries Society 102: 715–722.
Fowler, S. L., T. M. Reed & F. A. Dipper, 1997. The IUNC Species Survival Commission, No. 25: Elasmobranch Biodiversity Conservation and Management. Proceedings of the International Seminar and Workshop, Sabah, Malaysia, July 1997.
Fry, F. E. J, 1947. Effects of the environment on animal activity. University of Toronto Studies, Biological Series 55. Publication of the Ontario Fisheries Research Laboratory, Vol. 68: 1–62.
Fry, F. E. J., 1971. The Effect of Environmental Factors on the Physiology of Fish. Fish physiology. Academic Press, New York.
Garrone-Neto, D. & I. Sazima, 2009. Stirring, charging, and picking: hunting tactics of potamotrygonid rays in the upper Paraná River. Neotropical Ichthyology 7: 113–116.
Gebhart, G. E. & R. C. Summerfelt, 1978. Seasonal growth rates of fishes in relation to conditions of lake stratification. Proceedings of the Oklahoma Academy of Sciences 58: 6–10.
Hochachka, P. W. & G. N. Somero, 1973. Strategies of Biochemical Adaptation. W. B. Saunders Co., Philadelphia.
Hochachka, P. W. & G. N. Somero, 2002. Biochemical Adaptation: Mechanism and Process in Physiological Evolution. Oxford University Press, New York.
Hopkins, T. E. & J. J. Cech Jr, 1994. Effect of temperature on oxygen consumption of the bat ray, Myliobatis californica (Chondrichthyes, Mylobatididae). Copeia 1994: 529–532.
Huffard, C. L., 2007. Ethogram of Abdopus Aculeatus (D’orbigny, 1834) (Cephalopoda: Octopodidae): can behavioural characters inform octopodid taxomony & systematics? Journal of Molluscan Studies 73: 185–193.
IPCC, 2007. Climate change 2007: the physical science basis. In Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge.
IUCN, 2011. IUCN Red List of Threatened Species. Version 2011.2 [available on internet at www.iucnredlist.org].
Last, P. R. & J. D. Stevens, 1994. Sharks and Rays of Australia, 2nd ed. CSIRO Publishing, Melbourne.
Lugendo, B. R., I. Nagelkerken, N. Jiddawi, Y. D. Mgaya & G. Van Der Velde, 2007. Fish community composition of a tropical nonestuarine embayment in Zanzibar, Tanzania. Fisheries Science 73: 1213–1223.
Lutterschmidt, W. I. & V. H. Hutchison, 1997. The critical thermal maximum: data to support the onset of muscle spasm as the definitive end point. Canadian Journal of Zoology 75: 1553–1560.
Matern, S. A., J. J. Cech Jr & T. E. Hopkins, 2000. Diel movements of bat rays, Myliobatis californica, in Tomales Bay, California: evidence for behavioral thermoregulation? Environmental Biology of Fishes 58: 173–182.
McCauley, R. W. & N. W. Huggins, 1979. Ontogenetic and non-thermal seasonal effects on thermal preferenda of fish. American Zoologist 19: 267–271.
Meloni, C. J., J. J. Cech & S. M. Katzman, 2002. Effect of brackish salinities on oxygen consumption of bat rays (Myliobatis californica). Copeia 2002: 462–465.
Michael, S. W., 1993. Reef Sharks & Rays of the World: A guide to Their identification, Behavior, and Ecology. Sea Challengers, Monteray.
Munday, P. L., G. P. Jones, M. S. Pratchett & A. J. Williams, 2008. Climate change and the future for coral reef fishes. Fish and Fisheries 9: 261–285.
Meysman, F. J. R., J. J. Middelburg & C. H. R. Hiep, 2006. Bioturbation: a fresh look at Darwin’s last idea. TRENDS in Ecology and Evolution 21: 688–695.
Neer, J. A., J. K. Carlson & B. A. Thompson, 2006. Standard oxygen consumption of seasonally acclimatized cownose rays, Rhinoptera bonasus (Mitchill 1815), in the Northern Gulf of Mexico. Fish Physiology and Biochemistry 32: 67–71.
Nestler, J. M., R. A. Goodwin, T. M. Cole, D. Degan & D. Dennerline, 2002. Simulating movement patterns of blueback herring in a stratified southern impoundment. Transactions of the American Fisheries Society 131: 55–69.
Newell, R. C. & H. R. Northcroft, 1967. A reinterpretation of the effect of temperature on the metabolism of certain marine invertebrates. Journal of Zoology 151: 277–298.
Nguyen, N. T. & V. Q. Nguyen, 2006. Biodiversity and living resources of the coral reef fishes in Vietnam marine waters. Science and Technology Publishing House, Hanoi.
O’Shea, O. R., M. Thums, M. van Keulen & M. Meekan, 2011. Bioturbation by stingrays at Ningaloo Reef, Western Australia. Marine and Freshwater Research 62: 1323–1650.
Paladino, R. V., J. R. Spotila, J. P. Schubauer & K. T. Kowalski, 1980. The critical thermal maximum: a technique used to elucidate physiological stress and adaptation in fishes. Revue Canadienne de Biologie 39: 115–122.
Perry, A. L., P. J. Low, J. R. Ellis & J. D. Reynolds, 2005. Climate change and distribution shifts in marine fishes. Science 308: 1912–1915.
Poloczanska, E. S., R. C. Babcock, A. Butler, A. J. Hobday, O. Hoegh-Guldberg, T. J. Kunz, et al., 2007. Climate change and Australian marine life. In Gibson, R. N., J. D. M. Gordon & R. J. A. Atkinson (eds), Oceanography and Marine Biology. Taylor & Francis, New York: 407–478.
Portner, H. O. & A. P. Farrell, 2008. Physiology and climate change. Science 322: 690–692.
Portner, H. O. & R. Knust, 2007. Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science 315: 95–97.
Reber, C. S. & W. A. Bennett, 2007. The influence of thermal parameters on the acclimation responses of pinfish, Lagodon rhomboides, exposed to decreasing temperatures. The Journal of Fish Biology 71: 833–841.
Reynolds, W. W. & M. E. Casterlin, 1979. Behavioral thermoregulation and the “final preferendum” paradigm. American Zoologist 19: 211–224.
Rowe, D. K. & B. L. Chisnall, 1995. Effects of oxygen, temperature and light gradients on the vertical distribution of rainbow trout, Oncorhynchus mykiss, in two North Island, New Zealand, lakes differing in trophic status. New Zealand Journal of Marine and Freshwater Research 29: 421–434.
Schmidt-Nielsen, K., 1997. Animal Physiology: Adaptation and environment, 5th ed. Cambridge University Press, Cambridge.
Schulte, P. M., T. M. Healy & N. A. Fangue, 2011. Thermal performance curves, phenotypic plasticity, and the time scales of temperature exposure. Integrative and Comparative Biology 51: 691–702.
Sims, D. W., V. J. Wearmouth, E. J. Southall, J. M. Hill, P. Moore, K. Rawlinson, et al., 2006. Hunt warm, rest cool: bioenergetic strategy underlying diel vertical migration of a benthic shark. Journal of Animal Ecology 75: 176–190.
Steffensen, J. F., 1989. Some errors in respirometry of aquatic breathers: how to avoid and correct for them. Fish Physiology and Biochemistry 6: 49–59.
Stevens, E. D., 1992. Use of plastic materials in oxygen-measuring systems. Journal of Applied Physiology 72: 801–804.
Sumner, F. B. & P. Doudoroff, 1938. Some experiments upon temperature acclimatization and respiratory metabolism in fishes. Biological Bulletin 74: 403–429.
Taylor, J. R., M. M. Cook, A. L. Kirkpatrick, S. N. Galleher, J. Eme & W. A. Bennett, 2005. Thermal tactics of air-breathing and non air-breathing gobiids inhabiting mangrove tidepools on Pulau Hoga, Sulawesi, Indonesia. Copeia 2005: 886–893.
Teh, L., Al S Cabanban & U. R. Sumaila, 2005. The reef fisheries of Pulau Banggi, Sabah: a preliminary profile and assessment of ecological and socio-economic sustainability. Fisheries Research 76: 359–367.
Vaudo, J. J. & C. G. Lowe, 2006. Movement patterns of the round stingray Urobatis halleri (Cooper) near a thermal outfall. Journal of Fish Biology 68: 1756–1766.
Vonk, J. A., M. J. A. Christianen & J. Stapel, 2008. Redefining the trophic importance of seagrasses for fauna in tropical Indo-Pacific meadows. Estuarine, Coastal and Shelf Science 79: 653–660.
Wallman, H. L. & W. A. Bennett, 2006. Effects of parturition and feeding on thermal preference of Atlantic stingray, Dasyatis sabina. Environmental Biology of Fishes 75: 261–270.
White, W. T. & F. Dharmadi, 2007. Species and size compositions and reproductive biology of rays (Chondrichthyes, Batoidea) caught in target and non-target fisheries in eastern Indonesia. Journal of Fish Biology 70: 1809–1837.
Wilson, S. K., M. Adjeroud, D. R. Bellwood, M. L. Berumen, D. Booth, Y.-M. Bozec, P. Chabanet, et al., 2010. Crucial knowledge gaps in current understanding of climate change impacts on coral reef fishes. Journal of Experimental Biology 213: 894–900.
Zale, A. V., J. D. Wiechman, R. L. Lochmiller & J. Burroughs, 1990. Limnological conditions associated with summer mortality of striped bass in Keystone Reservoir, Oklahoma. Transactions of the American Fisheries Society 119: 72–76.
Acknowledgments
We thank Operation Wallacea, University of West Florida Research and Sponsored Programs, the Department of Biology, and the University of California Agricultural Experiment Station (grant no. 2098-H to N.A.F.) for providing funding. All animals in this study were treated in accordance with guidelines approved by the University of West Florida Animal Care and Use Committee (Protocol #2010-004).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: I. A. Nagelkerken
Rights and permissions
About this article
Cite this article
Dabruzzi, T.F., Bennett, W.A., Rummer, J.L. et al. Juvenile Ribbontail Stingray, Taeniura lymma (Forsskål, 1775) (Chondrichthyes, Dasyatidae), demonstrate a unique suite of physiological adaptations to survive hyperthermic nursery conditions. Hydrobiologia 701, 37–49 (2013). https://doi.org/10.1007/s10750-012-1249-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-012-1249-z