Abstract
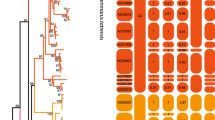
In this study, the mtDNA COI genes of 124 individuals in Brachionus calyciflorus complex were sequenced and analyzed. Overall, 84 mtDNA haplotypes were defined, and were split into three clades by the phylogenetic trees. The divergences of COI gene sequence among the three clades ranged from 11.4 to 22.5%, indicating the occurrence of three cryptic species (cryptic species 1, cryptic species 2, and cryptic species 3). Within cryptic species 3, a remarkable degree of differentiation (F st = 0.0947) might be attributable to habitat fragmentation, restricted gene flow, and founder effect, most probably together with local adaptation. The more shared haplotypes were observed, and a non-significant correlation existed between geographic and genetic distance, suggesting that some ancestral haplotypes might be involved in a past range expansion, and as founders give a similar distribution pattern among geographic populations. The networks were also in agreement with the pattern of genetic differentiation in B. calyciflorus species complex revealed by molecular phylogeny. The nested clade analysis suggested a slight geographic structure in the network I, network II, and the highest nesting clade levels within the network III. Under the possible effect of the Younger Dryas Event, the three cryptic species might have survived in multiple relict refugia throughout the south of China. The co-occurrence of cryptic species 2 and 3 might be found in Guangzhou and Hainan due to the possible interference of the barrier and contiguous range expansion. After the Younger Dryas Event, cryptic species 3 with high colonization ability might have arrived and colonized whole habitats at first, and reduce effective gene flow among cryptic species, thus effectively increasing persistence of founder events. Cryptic species 2 might, respectively, have expanded their ranges into Wuhu and Nanjing regions only because of the probable barrier of the Yangtze River, its weak colonization abilities and the ‘priority effects’ of cryptic species 3. Cryptic species 1 distributed exclusively in Danzhou probably failed to expand its ranges into mainland China because of small population size, weaker dispersal capacity, and the barrier of the Qiongzhou Strait.





Similar content being viewed by others
References
Allendorf, F. & N. Ryman, 2002. The role of genetics in population viability analysis. In Beissinger, S. R. & D. R. McCullough (eds), Population Viability Analysis. Wiley, New York.
Avise, J. C., 2009. Phylogeography: retrospect and prospect. Journal of Biogeography 36: 3–15.
Avise, J. C. & D. E. Walker, 1999. Species realities and numbers in sexual invertebrates: Perspectives from an asexually transmitted genome. Proceedings of the National Academy of Sciences of the United States of America 96: 992–995.
Bandelt, H. J., P. Forster & A. Röhl, 1999. Median-joining networks for inferring intraspecific phylogenies. Molecular Biology and Evolution 16: 37–48.
Bazin, E., S. Glémin & N. Galtier, 2006. Population size does not influence mitochondrial genetic diversity in animals. Science 5773: 570–572.
Boileau, M. G., P. D. N. Hebert & S. S. Schwartz, 1992. Non-equilibrium gene frequency divergence: persistent founder effects in natural populations. Journal of Evolutionary Biology 5: 25–39.
Cheng, X. F., Y. L. Xi & H. B. Li, 2008. Seasonal changes in the genetic structure of a Brachionus calyciflorus population in Lake Liantang based on its sequences. Acta Zoologica Sinica 54: 245–255.
Cox, A. J. & P. D. N. Hebert, 2001. Colonization, extinction, and phylogeographic patterning in a freshwater crustacean. Molecular Ecology 10: 371–386.
Crandall, K. A. & A. R. Templeton, 1993. Empirical tests of some predictions from coalescent theory with applications to intraspecific phylogeny reconstruction. Genetics 134: 959–969.
De Gelas, K. & L. De Meester, 2005. Phylogeography of Daphnia magna in Europe. Molecular Ecology 14: 753–764.
De Meester, L., 1993. Inbreeding and outbreeding depression in Daphnia. Oecologia 96: 80–84.
De Meester, L., 1996. Local genetic differentiation and adaptation in freshwater zooplankton populations: patterns and processes. Ecoscience 3: 385–399.
De Meester, L., A. Gómez, B. Okamura & K. Schwenk, 2002. The monopolization hypothesis and the dispersal–gene flow paradox in aquatic organisms. Acta Oecologica 23: 121–135.
Derry, A. M., P. D. N. Hebert & E. E. Prepas, 2003. Evolution of rotifers in saline and subsaline lakes: a molecular phylogenetic approach. Limnology and Oceanography 48: 675–685.
Dodson, S. I., A. K. Grishanin, K. Gross & G. A. Wyngaard, 2003. Morphological analysis of some cryptic species in the Acanthocyclops vernalis species complex from North America. Hydrobiologia 500: 131–143.
Dong, Y. W. & C. J. Niu, 2004. Sequence variability of mitochondrial COI region and population genetic structure of rotifer Brachionus calyciflorus. Oceanologia et Limnologia Sinica 35: 473–480.
Excoffier, L. G. L. & S. Schneider, 2005. Arlequin ver. 3.0: an integrated software package for population genetics data analysis. Evolutionary Bioinformatics Online 1: 47–50.
Felsenstein, J., 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39: 783–791.
Folmer, O., M. Black, W. Hoeh, R. Lutz & R. Vrijenhoek, 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology 3: 294–299.
Freeland, J. R., L. R. Noble & B. Okamura, 2000. Genetic diversity of North American populations of Cristatella mucedo, inferred from microsatellite and mitochondrial DNA. Molecular Ecology 9: 1375–1389.
Gilbert, J. J., 1963. Mictic female production in the rotifer Brachionus calyciflorus. Journal of Experimental Zoology 153: 113–124.
Gilbert, J. J. & E. J. Walsh, 2005. Brachionus calyciflorus is a species complex: mating behavior and genetic differentiation among four geographically isolated strains. Hydrobiologia 546: 257–265.
Gómez, A., 1997. Ecological factors affecting gene flow in the Brachionus plicatilis complex (rotifera). Oecologia 111: 350–356.
Gómez, A., 2005. Molecular ecology of rotifers: from population differentiation to speciation. Hydrobiologia 546: 83–99.
Gómez, A., M. Temprano & M. Serra, 1995. Ecological genetics of a cyclical parthenogen in temporary habitats. Journal of Evolutionary Biology 8: 601–622.
Gómez, A., G. R. Carvalho & D. H. Lunt, 2000. Phylogeography and regional endemism of a passively dispersing zooplankter: mitochondrial DNA variation in rotifer resting egg banks. Proceedings of the Royal Society B: Biological Sciences 267:2189–2197.
Gómez, A., G. J. Adcock, D. H. Lunt & G. R. Carvalho, 2002a. The interplay between colonization history and gene flow in passively dispersing zooplankton: microsatellite analysis of rotifer resting egg banks. Journal of Evolutionary Biology 15: 158–171.
Gómez, A., M. Serra, G. R. Carvalho & D. H. Lunt, 2002b. Speciation in ancient cryptic species complexes: evidence from the molecular phylogeny of Brachionus plicatilis (rotifera). Evolution 56: 1431–1444.
Gómez, A., J. Montero-pau, D. H. Lunt, M. Serra & S. Campillo, 2007. Persistent genetic signatures of colonization in Brachionus manjavacas rotifers in the Iberian Peninsula. Molecular Ecology 16: 3228–3240.
Hairston, N. G., 1996. Zooplankton egg banks as biotic reservoirs in changing environments. Limnology and Oceanography 41: 1087–1092.
Hao, J. S., Q. Yang, C. Li, K. Zhang & X. Sun, 2003. Molecular phylogeny of lophotrochozoa based on 18S rRNA gene sequences-with comment on the phylogenetic position of bryozoa. Journal of genetics and molecular biology 14: 64–72.
Hatton-Ellis, T. W., L. R. Noble & B. Okamura, 1998. Genetic variation in a freshwater bryozoan. I: Populations in the Thames basin, UK. Molecular Ecology 7: 1575–1585.
Hebert, P. D. N., 1987. Genetics of Daphnia. In Peters, R. H. & R. De Bernardi (eds), The Biology of Daphnia. Memorie Istitute Italiano Di Idrobiologia, 45: 439–460.
Hebert, P. D. N., 1998. Advances in molecular ecology: variable environments and evolutionary diversification in inland waters. IOS Press, Amsterdam.
Hebert, P. D. N. & B. J. Hann, 1986. Patterns in the composition of arctic tundra pond microcrustacean communities. Canadian Journal of Fisheries and Aquatic Sciences 43: 1416–1425.
Hebert, P. D. N. & C. Wilson, 1994. Provincialism in plankton: endemism and allopatric speciation in Australian Daphnia. Evolution 48: 1333–1349.
Hebert, P. D. N., J. D. S. Witt & S. J. Adamowicz, 2003. Phylogeographical patterning in Daphnia ambigua: regional divergence and intercontinental cohesion. Limnology and Oceanography 48: 261–268.
Hutchinson, G. E., 1967. A treatise on limnology II. Introduction to lake biology and limnoplankton. Wiley, New York.
Innes, D. J., 1991. Geographic patterns of genetic differentiation among sexual populations of Daphnia pulex. Canadian Journal Zoology 69: 995–1003.
Ishida, S. & D. J. Taylor, 2007. Mature habitats associated with genetic divergence despite strong dispersal ability in an arthropod. BMC Evolutionary Biology 7: 52.
Kim, K., A. A. Kotov & D. J. Taylor, 2006. Hormonal induction of undescribed males resolves cryptic species of cladocerans. Proceedings of the Royal Society of London Series B 273:141–147.
King, C. E. & M. Serra, 1998. Seasonal variation as a determinant of population structure in rotifers reproducing by cyclical parthenogenesis. Hydrobiologia 387(388): 361–372.
Knowlton, N., 1993. Sibling species in the sea. Annual Review of Ecology and Systematics 24: 189–216.
Knowlton, N., 2000. Molecular genetic analyses of species boundaries in the sea. Hydrobiologia 420: 73–90.
Lee, C. E., 2000. Global phylogeography of a cryptic copepod species complex and reproductive isolation between genetically proximate ‘populations’. Evolution 54: 2014–2027.
Lee, C. E. & B. W. Frost, 2002. Morphological stasis in the Eurytemora affinis species complex (Copepoda: Temoridae). Hydrobiologia 480: 111–128.
Li, S. H., H. Zhu, Y. Z. Xia, M. J. Yu, K. S. Liu, Z. Y. Ye & Y. X. Chen, 1959. The mass culture of unicellular green algae. Acta Hydrobiologica Sinica 4: 462–472.
Li, H. B., Y. L. Xi, X. F. Cheng, X. L. Xiang, C. B. Hu & L. X. Tao, 2008. Sympatric speciation in rotifers: evidence from molecular phylogenetic relationships and reproductive isolation among Brachionus calyciflorus clones. Acta Zoologica Sinica 54: 256–264.
Lowe, C. D., S. J. Kemp, C. Diaz-Avalos & D. J. S. Montagnes, 2007. How does salinity tolerance influence the distributions of Brachionus plicatilis sibling species? Marine Biology 150: 377–386.
Mantel, N., 1967. The detection of disease clustering and a generalized regression approach. Cancer Research 27: 209–220.
Mergeay, J., D. Verschuren & L. De Meester, 2005. Cryptic invasion and dispersal of an American Daphnia in East Africa. Limnology and Oceanography 50: 1278–1283.
Mills, S., D. H. Lunt & A. Gomez, 2007. Global isolation by distance despite strong regional phylogeography in a small metazoan. BMC Evolutionary Biology 7: 225.
Muñoz, J., A. Gómez, A. J. Green, J. Figuerola, F. Amat & C. Rico, 2008. Phylogeography and local endemism of the native Mediterranean brine shrimp Artemia salina (Branchiopoda: Anostraca). Molecular Ecology 17: 3160–3177.
Nei, M. & W. H. Li, 1979. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proceedings of the National Academy of Sciences of the United States of America 76:5269–5273.
Okamura, B. & J. Freeland, 2002. Gene flow and the evolutionary ecology of passively dispersing aquatic invertebrates. In Bullock, J. M., R. E. Kenward & R. S. Hails (eds), Dispersal. Blackwell Scientific Publications, London.
Ortells, R., T. W. Snell, A. Gomez & M. Serra, 2000. Patterns of genetic differentiation in resting egg banks of a rotifer species complex in Spain. Archiv fuer Hydrobiologie 149: 529–551.
Ortells, R., A. Gomez & M. Serra, 2003. Coexistence of cryptic rotifer species: ecological and genetic characterisation of Brachionus plicatilis. Freshwater Biology 48: 2194–2202.
Penton, E. H., P. D. N. Hebert & T. J. Crease, 2004. Mitochrondrial DNA variation in North American populations of Daphnia obtusa: continentalism or cryptic endemism? Molecular Ecology 13: 97–107.
Posada, D. & K. A. Crandall, 1998. Modeltest: testing the model of DNA substitution. Bioinformatics 9: 817–818.
Posada, D., K. A. Crandall & A. R. Templeton, 2000. GeoDis: a program for the cladistic nested analysis of the geographical distribution of genetic haplotypes. Molecular Ecology 9: 487–488.
Ronquist, F. & J. P. Huelsenbeck, 2003. Mrbayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574.
Rozas, J., J. C. Sánchez-Delbarrio, X. Messeguer & R. Rozas, 2003. Dnasp, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics 19: 2496–2497.
Schon, I., A. Gandolfi, E. Di Masso, V. Rossi, H. I. Griffiths, K. Martens & R. K. Butlin, 2000. Persistence of asexuality through mixed reproduction in Eucypris virens (Crustacea, Ostracoda). Heredity 84: 161–169.
Schröder, T. & E. J. Walsh, 2007. Cryptic speciation in the cosmopolitan Epiphanes senta complex (Monogononta, Rotifera) with the description of new species. Hydrobiologia 593: 129–140.
Stemberger, R. S., 1995. Pleistocene refuge areas and postglacial dispersal of copepods of the northeastern United States. Canadian Journal of Fisheries and Aquatic Sciences 52: 2197–2210.
Suatoni, E., 2003. Patterns of speciation in the rotifer species complex, Brachionus plicatilis. Yale University, New Haven: 115.
Swofford, D. L., 2002. Phylogenetic analysis using parsimony (*and other methods). Sinauer Associates, Sunderland.
Taylor, D. J. & P. D. N. Hebert, 1992. Daphnia galeata mendotae as a cryptic species complex with interspecific hybrids. Limnology and Oceanography 37: 658–665.
Taylor, D. J., T. L. Finston & P. D. N. Hebert, 1998. Biogeography of a widespread freshwater crustacean: pseudocongruence and cryptic endemism in the North American Daphnia leavis complex. Evolution 52: 1648–1670.
Templeton, A. R. & C. F. Sing, 1993. A cladistic analysis of phenotypic associations with haplotypes inferred from restriction endonuclease mapping. IV. Nested analysis with cladogram uncertainty and recombination. Genetics 134: 659–669.
Templeton, A. R., E. Boerwinkle & C. F. Sing, 1987. A cladistic analysis of phenotypic associations with haplotypes inferred from restriction endonuclease mapping. I. Basic theory and an analysis of alcohol dehydrogenase activity in Drosophila. Genetics 117: 343–351.
Templeton, A. R., K. A. Crandall & C. F. Sing, 1992. A cladistic analysis of phenotypic associations with haplotypes inferred from restriction endonuclease mapping and DNA sequence data. III. Cladogram estimation. Genetics 132: 619–633.
Templeton, A. R., E. Routman & C. A. Phillips, 1995. Separating population structure from population history: a cladistic analysis of the geographical distribution of mitochondrial DNA haplotypes in the Tiger Salamander, Ambystoma tigrinurn. Genetics 140: 767–782.
Thomas, J. A., J. J. Welch, M. Woolfit & L. Bromham, 2006. There is no universal molecular clock for invertebrates, but rate variation does not scale with body size. Proceedings of the National Academy of Sciences of the United States of America 103:7366–7371.
Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin & D. G. Higgins, 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25(24): 4876–4882.
Vanoverbeke, J. & L. De Meester, 1997. Among-populational genetic differentiation in the cyclical parthenogen Daphnia magna (Crustacea, Anomopoda) and its relation to geographic distance and clonal diversity. Hydrobiologia 360: 135–142.
Weider, L. J., A. Hobæk, J. K. Colbourne, T. J. Crease, F. Dufresne & P. D. N. Hebert, 1999a. Holoarctic phylogeography of an asexual species complex. I. Mitochondrial DNA variation in arctic Daphnia. Evolution 53: 777–792.
Weider, L. J., A. Hobaek, P. D. N. Hebert & T. J. Crease, 1999b. Holarctic phylogeography of an asexual species complex. II. Allozymic variation and clonal structure in arctic Daphnia. Molecular Ecology 8: 1–13.
Xiao, J. Y., J. Wang, Z. S. An, X. H. Wu & W. J. Zhou, 1998. Evidence for the Younger Dryas Event in the eastern part of Nanling Region. Acta Botanica Sinica 40: 1079–1082.
Yu, Y., Q. Yan & W. Feng, 2008. Spatio-temporal heterogeneity of plankton communities in lake Donghu, China, as revealed by PCR-denaturing gradient gel electrophoresis and its relation to biotic and abiotic factors. FEMS Microbiology Ecology 63: 328–337.
Acknowledgments
This research was funded by Natural Science Foundation of China (30770352, 30499341), Excellent Youth Foundation in Anhui Province (08040106904), Natural Science Foundation of Educational Committee of Anhui Province (KJ2009B089Z), Foundation of Provincial Key Laboratory of Biotic Environment and Ecological Safety in Anhui (2004sys003), the Foundation of Provincial Key Laboratory of Conservation and Utilization for Important Biological Resource in Anhui, and the Grant for Youth of Anhui Normal University (2008xqn71, 2007xqn74).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Koen Martens
Rights and permissions
About this article
Cite this article
Xiang, XL., Xi, YL., Wen, XL. et al. Spatial patterns of genetic differentiation in Brachionus calyciflorus species complex collected from East China in summer. Hydrobiologia 638, 67–83 (2010). https://doi.org/10.1007/s10750-009-0010-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-009-0010-8