Abstract
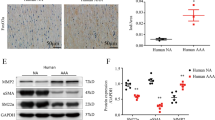
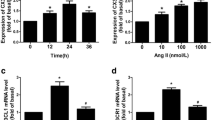
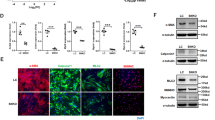
The development of acute aortic dissection (AD) is attributed to unbearable wall tension superimposed on disordered of cells and extracellular matrix (ECM) in the aortic wall. Adventitial fibroblasts (AFs) phenotypic differentiation response to stress exhibits essential function to regulate the remolding of vascular. Little is known about the AFs phenotypic differentiation and its possible mechanism in patients with AD. In this study, we examined their roles in AD. Surgical specimens of the aorta from AD patients (n = 10) and controls (n = 10) were tested for α-smooth muscle actin (α-SMA), extracellular signal-regulated kinase 1,2 (ERK1/2) and phospho-ERK1/2 expression, respectively by western blot. When compared with controls, protein levels of α-SMA was significantly decreased and levels of phospho-ERK1/2 was increased significantly in the aortic wall from patients with AD. Immunohistochemistry results showed elevated staining of both α-SMA and phospho-ERK1/2 in the adventitia of the aortic wall from patients with AD, on the contrary, staining of α-SMA in the media was decreased compared with controls. In vitro, the Raf/MEK/ERK pathway was involved in Ang-II-induced phenotypic differentiation and matrix metalloproteinase-2 (MMP-2) mRNA expression in AFs. This study provides a new insight into the biological action of AFs and phospho-ERK1/2 promoting phenotypic differentiation and MMP-2 expression, suggesting an important role of AFs in leading to disorder the delicate balance of ECM metabolism in the aortic wall, so that AFs may be an essential participant during AD formation.




Similar content being viewed by others
References
Aimes RT, Quigley JP (1995) Matrix metalloproteinase-2 is an interstitial collagenase. Inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the specific 3/4- and 1/4-length fragments. J Biol Chem 270(11):5872–5876
Akiyama M et al (2006) Up-regulation of matrix metalloproteinase-2 and membrane-type 1-matrix metalloproteinase were coupled with that of type I procollagen in granulation tissue response after the onset of aortic dissection. Virchows Archiv 448:811–821
Allaire E et al (2009) New insight in aetiopathogenesis of aortic diseases. Eur J Vasc Endovasc Surg 37(5):531–537
Arenas IA et al (2004) Angiotensin II-induced MMP-2 release from endothelial cells is mediated by TNF-alpha. Am J Physiol Cell Physiol 286(4):C779–C784
Barker SG et al (1994) The adventitia and atherogenesis: removal initiates intimal proliferation in the rabbit which regresses on generation of a ‘neoadventitia’. Atherosclerosis 105(2):131–144
Belknap JK et al (1997) Hypoxia increases bromodeoxyuridine labeling indices in bovine neonatal pulmonary arteries. Am J Respir Cell Mol Biol 16(4):366–371
Beltran AE et al (2009) p38 MAPK contributes to angiotensin II-induced COX-2 expression in aortic fibroblasts from normotensive and hypertensive rats. J Hypertens 27(1):142–154
Castoldi G et al (2007) Angiotensin II increases tissue-specific inhibitor of metalloproteinase-2 expression in rat aortic smooth muscle cells in vivo: evidence of a pressure-independent effect. Clin Exp Pharmacol Physiol 34(3):205–209
Coady MA et al (1999) Natural history, pathogenesis, and etiology of thoracic aortic aneurysms and dissections. Cardiol Clin 17(4):615–635
Daugherty A, Cassis LA (2004) Mouse models of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol 24(3):429–434
Daugherty A, Manning MW, Cassis LA (2000) Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest 105(11):1605–1612
Davis V et al (1998) Matrix metalloproteinase-2 production and its binding to the matrix are increased in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol 18(10):1625–1633
Freestone T et al (1995) Inflammation and matrix metalloproteinases in the enlarging abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol 15(8):1145–1151
Gabbiani G (2003) The myofibroblast in wound healing and fibrocontractive diseases. J Pathol 200(4):500–503
Ghosh A et al (2012) The role of extracellular signal-related kinase during abdominal aortic aneurysm formation. J Am Coll Surg 215(5):668–680
Grotendorst GR, Rahmanie H, Duncan MR (2004) Combinatorial signaling pathways determine fibroblast proliferation and myofibroblast differentiation. FASEB J 18(3):469–479
Hagan PG et al (2000) The international registry of acute aortic dissection (IRAD): new insights into an old disease. JAMA 283(7):897–903
Hinz B et al (2007) The myofibroblast: one function, multiple origins. Am J Pathol 170(6):1807–1816
Hirst AJ, Johns VJ, Kime SJ (1958) Dissecting aneurysm of the aorta: a review of 505 cases. Medicine (Baltimore) 37(3):217–279
Huang J et al (2010) Fibulin-4 deficiency results in ascending aortic aneurysms: a potential link between abnormal smooth muscle cell phenotype and aneurysm progression. Circ Res 106(3):583–592
Ishii T, Asuwa N (2000) Collagen and elastin degradation by matrix metalloproteinases and tissue inhibitors of matrix metalloproteinase in aortic dissection. Hum Pathol 31(6):640–646
Ji J et al (2010) Activation of adventitial fibroblasts in the early stage of the aortic transplant vasculopathy in rat. Transplantation 89(8):945–953
Leung PS, Chan YC (2009) Role of oxidative stress in pancreatic inflammation. Antioxid Redox Signal 11(1):135–165
Li L et al (2006) Increased migration of vascular adventitial fibroblasts from spontaneously hypertensive rats. Hypertens Res 29(2):95–103
Liao S et al (2001) Suppression of experimental abdominal aortic aneurysms in the rat by treatment with angiotensin-converting enzyme inhibitors. J Vasc Surg 33(5):1057–1064
Luchtefeld M et al (2005) Angiotensin II induces MMP-2 in a p47phox-dependent manner. Biochem Biophys Res Commun 328(1):183–188
Milewicz DM et al (2008) Genetic basis of thoracic aortic aneurysms and dissections: focus on smooth muscle cell contractile dysfunction. Ann Rev Genomics Hum Genet 9:283–302
Nagashima H et al (2001) Angiotensin II type 2 receptor mediates vascular smooth muscle cell apoptosis in cystic medial degeneration associated with Marfan’s syndrome. Circulation 104(12 Suppl 1):I282–I287
Nishijo N et al (1998) Salt-sensitive aortic aneurysm and rupture in hypertensive transgenic mice that overproduce angiotensin II. Lab Invest 78(9):1059–1066
Pearson G et al (2001) Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev 22(2):153–183
Peng J et al (2002) Tumor necrosis factor-alpha-induced AT1 receptor upregulation enhances angiotensin II-mediated cardiac fibroblast responses that favor fibrosis. Circ Res 91(12):1119–1126
Rayner K et al (2000) Localisation of mRNA for JE/MCP-1 and its receptor CCR2 in atherosclerotic lesions of the ApoE knockout mouse. J Vasc Res 37(2):93–102
Sakalihasan N, Limet R, Defawe OD (2005) Abdominal aortic aneurysm. Lancet 365(9470):1577–1589
Sakata N et al (2007) Possible involvement of myofibroblast in the development of inflammatory aortic aneurysm. Pathol Res Pract 203(1):21–29
Scott NA et al (1996) Identification of a potential role for the adventitia in vascular lesion formation after balloon overstretch injury of porcine coronary arteries. Circulation 93(12):2178–2187
Shen WL et al (2006) NAD(P)H oxidase-derived reactive oxygen species regulate angiotensin-II induced adventitial fibroblast phenotypic differentiation. Biochem Biophys Res Commun 339(1):337–343
Shi Y et al (1996) Adventitial myofibroblasts contribute to neointimal formation in injured porcine coronary arteries. Circulation 94(7):1655–1664
Shi-Wen X et al (2004) Endothelin-1 promotes myofibroblast induction through the ETA receptor via a rac/phosphoinositide 3-kinase/Akt-dependent pathway and is essential for the enhanced contractile phenotype of fibrotic fibroblasts. Mol Biol Cell 15(6):2707–2719
Tang MJ et al (1998) Collagen gel overlay induces apoptosis of polarized cells in cultures: disoriented cell death. Am J Physiol 275(4 Pt 1):C921–C931
Tieu BC et al (2009) An adventitial IL-6/MCP1 amplification loop accelerates macrophage-mediated vascular inflammation leading to aortic dissection in mice. J Clin Invest 119(12):3637–3651
Touyz RM et al (1999) Role of extracellular signal-regulated kinases in angiotensin II-stimulated contraction of smooth muscle cells from human resistance arteries. Circulation 99(3):392–399
Touyz RM et al (2004) Angiotensin II and endothelin-1 regulate MAP kinases through different redox-dependent mechanisms in human vascular smooth muscle cells. J Hypertens 22(6):1141–1149
Tsunemi K et al (2002) Possible roles of angiotensin II-forming enzymes, angiotensin converting enzyme and chymase-like enzyme, in the human aneurysmal aorta. Hypertens Res 25(6):817–822
Tsuruda T et al (2004) Adrenomedullin induces matrix metalloproteinase-2 activity in rat aortic adventitial fibroblasts. Biochem Biophys Res Commun 325(1):80–84
Wang X et al (2006) Increased collagen deposition and elevated expression of connective tissue growth factor in human thoracic aortic dissection. Circulation 114(1 Suppl):I200–I205
Wang HD et al (2010) Adventitial fibroblasts in vascular structure and function. Canad J Physiol Pharmacol 88(3):177–186
White M, Montezano AC, Touyz RM (2012) Angiotensin II signalling and calcineurin in cardiac fibroblasts: differential effects of calcineurin inhibitors FK506 and cyclosporine A. Ther Adv Cardiovasc Dis 6(1):5–14
Wilcox JN et al (2001) Perivascular responses after angioplasty which may contribute to postangioplasty restenosis: a role for circulating myofibroblast precursors? Ann N Y Acad Sci 947:68–90; discussion 90-2
Xu F et al (2007) Adventitial fibroblasts are activated in the early stages of atherosclerosis in the apolipoprotein E knockout mouse. Biochem Biophys Res Commun 352(3):681–688
Yang W et al (2011) Angiotensin II downregulates catalase expression and activity in vascular adventitial fibroblasts through an AT1R/ERK1/2-dependent pathway. Mol Cell Biochem 358(1–2):21–29
Zalewski A, Shi Y (1997) Vascular myofibroblasts. Lessons from coronary repair and remodeling. Arterioscler Thromb Vasc Biol 17(3):417–422
Acknowledgments
This work was supported by National Natural Science Foundation of China (grant number 30872537 to Zhiwei Wang) and Natural Science Foundation of Hubei Province of China (grant number 2008CHB421 and 2013CKB031 to Zhiwei Wang).
Author information
Authors and Affiliations
Corresponding author
Additional information
Zhiwei Wang and Zongli Ren contributed to the work equally and should be regarded as co-first authors.
Rights and permissions
About this article
Cite this article
Wang, Z., Ren, Z., Hu, Z. et al. Angiotensin-II induces phosphorylation of ERK1/2 and promotes aortic adventitial fibroblasts differentiating into myofibroblasts during aortic dissection formation. J Mol Hist 45, 401–412 (2014). https://doi.org/10.1007/s10735-013-9558-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-013-9558-8