Abstract
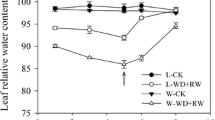
Effects of severity and duration of osmotic stress on partial root system were investigated on root hydraulic conductance (L p ) and growth in each sub-root system. The maize seedlings were raised in nutrient solution with the roots divided equally into two containers. At 12 days after transplanting, half of root system was subjected to osmotic stress of −0.2, −0.4 and −0.6 MPa using PEG 6000 added in the solution, and no PEG 6000 solution was taken as control (CK). L p and root growth in each sub-root system were measured at 0, 0.25, 0.5, 1, 3, 5, 7 and 9 days after treatment (DAT). Results show that compared to CK, −0.2 MPa treatment enhanced the hydraulic conductance per root area (L pr ) in non-stressed sub-root system by 12 % at 0.5 DAT. In −0.4 MPa treatment, L pr in non-stressed sub-root system was significantly increased at 9 DAT. But in −0.6 MPa treatment, significant decrease of 12–40 % was observed in non-stressed sub-root system during 0.5–3 DAT if compared to CK, indicating that the threshold of osmotic stress for the compensatory effect of water uptake in non-stressed sub-root system was between −0.2 and −0.4 MPa. In addition, the root area growth rate in non-stressed sub-root system was significantly higher than that in CK during 0.25–0.5, 1–3 and 5–7 DAT for −0.2, −0.4 and −0.6 MPa treatments, respectively. Similar trend also was observed for root length growth rate, indicating that osmotic stress could still stimulate the compensatory effect of root growth other than water uptake under partial root system. Moreover, the occurrence of such compensatory effect delayed with the increasing osmotic stress. Meanwhile, −0.2 MPa treatment had no significant effect on maize shoot growth but −0.4 and −0.6 MPa treatments inhibited shoot biomass accumulation, which resulted in higher root/shoot ratio in −0.4 and −0.6 MPa treatments. Thus root plasticity in hydraulic conductance and root growth in different root zones varied largely depending on the severity and duration of osmotic stress under partial root system.





Similar content being viewed by others
References
An Y, Zhang M, Liu G, Han R, Liang Z (2013) Proline accumulation in leaves of Periploca sepium via both biosynthesis up-regulation and transport during recovery from severe drought. PloS One 8:1–10
Aranda I, Alía R, Ortega U, Dantas Â, Majada J (2010) Intra-specific variability in biomass partitioning and carbon isotopic discrimination under moderate drought stress in seedlings from four Pinus pinaster populations. Tree Genet Genomes 6:169–178
Aroca R, Ferrante A, Vernieri P, Chrispeels MJ (2006) Drought, abscisic acid and transpiration rate effects on the regulation of PIP aquaporin gene expression and abundance in Phaseolus vulgaris plants. Ann Bot Lond 98:1301–1310
Banco DM, Yamauchi A, Kamoshita A, Wade LJ, Jose R, Pardales J (2000) Dry matter production and root system development of rice cultivars under fluctuating soil moisture. Plant Prod Sci 3:197–207
Ben-Asher J, Silberbush M (1992) Root distribution under trickle irrigation: factors affecting distribution and comparison among methods of determination. J Plant Nutr 15:783–794
Bramley H, Turner DW, Tyerman SD, Turner NC (2007) Water flow in the roots of crop species: the influence of root structure, aquaporin activity, and waterlogging. Adv Agron 96:133–196
Cahill JF Jr, McNickle GG (2011) The behavioral ecology of nutrient foraging by plants. Annu Rev Ecol Evol Syst 42:289–311
Caldwell M, Dudley L, Lilieholm B (1992) Soil solution phosphate, root uptake kinetics and nutrient acquisition: implications for a patchy soil environment. Oecologia 89:305–309
Chaves M, Oliveira M (2004) Mechanisms underlying plant resilience to water deficits: prospects for water-saving agriculture. J Exp Bot 55:2365–2384
Chien SH, Gearhart MM, Villagarcía S (2011) Comparison of ammonium sulfate with other nitrogen and sulfur fertilizers in increasing crop production and minimizing environmental impact: a review. Soil Sci 176:327–335
Davies WJ, Zhang J (1991) Root signals and the regulation of growth and development of plants in drying soil. Annu Rev Plant Biol 42:55–76
Deak KI, Malamy J (2005) Osmotic regulation of root system architecture. Plant J 43:17–28
Draye X, Kim Y, Lobet G, Javaux M (2010) Model-assisted integration of physiological and environmental constraints affecting the dynamic and spatial patterns of root water uptake from soils. J Exp Bot 61:2145–2155
Drew M, Nye P (1970) The supply of nutrient ions by diffusion to plant roots in soil: III. Uptake of phosphate by roots of onion, leek, and rye-grass. Plant Soil 33:545–563
Dry P, Loveys B, Düring H (2000) Partial drying of the root zone of grape. II. Changes in the pattern of root development. Vitis 39:9–12
Du N, Guo W, Zhang X, Wang R (2010) Morphological and physiological responses of Vitex negundo L. var. heterophylla (Franch.) Rehd. to drought stress. Acta Physiol Plant 32:839–848
Enstone DE, Peterson CA, Ma F (2002) Root endodermis and exodermis: structure, function, and responses to the environment. J Plant Growth Regul 21:335–351
Frensch J, Steudle E (1989) Axial and radial hydraulic resistance to roots of maize (Zea mays L.). Plant Physiol 91:719–726
Gallardo M, Turner N, Ludwig C (1994) Water relations, gas exchange and abscisic acid content of Lupinus cosentinii leaves in response to drying different proportions of the root system. J Exp Bot 45:909–918
Gullo M, Salleo S (1993) Different vulnerabilities of Quercus ilex L. to freeze-and summer drought-induced xylem embolism: an ecological interpretation. Plant Cell Environ 16:511–519
Hodge A, Stewart J, Robinson D, Griffiths B, Fitter A (2000) Spatial and physical heterogeneity of N supply from soil does not influence N capture by two grass species. Funct Ecol 14:645–653
Hsiao TC (1973) Plant responses to water stress. Annu Rev Plant Physiol 24:519–570
Hu T, Kang S, Yuan L, Zhang F, Li Z (2008) Effects of partial root-zone irrigation on growth and development of maize root system. Acta Ecol Sin 28:6180–6188
Hu TT, Kang SZ, Li FS, Zhang JH (2011) Effects of partial root-zone irrigation on hydraulic conductivity in the soil-root system of maize plants. J Exp Bot 62:4163–4172
Jackson RB, Sperry JS, Dawson TE (2000) Root water uptake and transport: using physiological processes in global predictions. Trends Plant Sci 5:482–488
Johansson I, Karlsson M, Shukla VK, Chrispeels MJ, Larsson C, Kjellbom P (1998) Water transport activity of the plasma membrane aquaporin PM28A is regulated by phosphorylation. Plant Cell 10(3):451–459
Kang S, Zhang J (2004) Controlled alternate partial root-zone irrigation: its physiological consequences and impact on water use efficiency. J Exp Bot 55:2437–2446
Lei Y, Yin C, Li C (2006) Differences in some morphological, physiological, and biochemical responses to drought stress in two contrasting populations of Populus przewalskii. Physiol Plant 127:182–191
Liang H, Li F, Nong M (2013) Effects of alternate partial root-zone irrigation on yield and water use of sticky maize with fertigation. Agric Water Manag 116:242–247
Lo Gullo MA, Nardini A, Salleo S, Tyree MT (1998) Changes in root hydraulic conductance (KR) of Olea oleaster seedlings following drought stress and irrigation. New Phytol 140:25–31
Lynch JP (2013) Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems. Ann Bot 112:347–357
Lynch JP, Brown KM (2008) Root strategies for phosphorus acquisition. In: White PJ, Hammond JP (eds) The ecophysiology of plant-phosphorus interactions. Springer, Netherlands, pp 83–116
Maurel C, Simonneau T, Sutka M (2010) The significance of roots as hydraulic rheostats. J Exp Bot 61:3191–3198
Mingo DM, Theobald JC, Bacon MA, Davies WJ, Dodd IC (2004) Biomass allocation in tomato (Lycopersicon esculentum) plants grown under partial root zone drying: enhancement of root growth. Funct Plant Biol 31:971–978
Miyamoto N, Steudle E, Hirasawa T, Lafitte R (2001) Hydraulic conductivity of rice roots. J Exp Bot 52:1835–1846
Mu Z-X, Liang Z-S, Zhang S-Q (2002) Physiological basis of compensation growth of crops under soil alternate drying-wetting and its application in agricultural produce. Plant Physiol Commun 38:511–516
Nacry P, Bouguyon E, Gojon A (2013) Nitrogen acquisition by roots: physiological and developmental mechanisms ensuring plant adaptation to a fluctuating resource. Plant Soil 370:1–29
Niones J, Suralta R, Inukai Y, Yamauchi A (2012) Field evaluation on functional roles of root plastic responses on dry matter production and grain yield of rice under cycles of transient soil moisture stresses using chromosome segment substitution lines. Plant Soil 359:107–120
North GB, Nobel PS (1992) Drought-induced changes in hydraulic conductivity and structure in roots of Ferocactus acanthodes and Opuntia ficus-indica. New Phytol 120:9–19
Okada K, Kondo M, Ando H, K-i Kakuda (2002) Water uptake under water stress at panicle initiation stage in upland rice as affected by previous soil water regimes. Soil Sci Plant Nutr 48:151–158
Perumalla C, Peterson CA (1986) Deposition of Casparian bands and suberin lamellae in the exodermis and endodermis of young corn and onion roots. Can J Bot 64:1873–1878
Rieger M (1995) Offsetting effects of reduced root hydraulic conductivity and osmotic adjustment following drought. Tree Physiol 15:379–385
Romero P, Dodd IC, Martinez-Cutillas A (2012) Contrasting physiological effects of partial root zone drying in field-grown grapevine (Vitis vinifera L. cv. Monastrell) according to total soil water availability. J Exp Bot 63:4071–4083
Sampathkumar T, Pandian BJ, Mahimairaja S (2012) Soil moisture distribution and root characters as influenced by deficit irrigation through drip system in cotton–maize cropping sequence. Agric Water Manag 103:43–53
Schreiber L, Hartmann K, Skrabs M, Zeier J (1999) Apoplastic barriers in roots: chemical composition of endodermal and hypodermal cell walls. J Exp Bot 50:1267–1280
Schumacher TE, Smucker AJM (1987) Ion uptake and respiration of dry bean roots subjected to localized anoxia. Plant Soil 99:411–422
Schurr U, Gollan T, Schulze ED (1992) Stomatal response to drying soil in relation to changes in the xylem sap composition of Helianthus annuus. II. Stomatal sensitivity to abscisic acid imported from the xylem sap. Plant Cell Environ 15(5):561–567
Steudle E (2000) Water uptake by roots: effects of water deficit. J Exp Bot 51:1531–1542
Suralta R, Inukai Y, Yamauchi A (2008) Genotypic variations in responses of lateral root development to transient moisture stresses in rice [Oryza sativa] cultivars. Plant Prod Sci (Japan) 11(3):324–335
Tezara W, Mitchell V, Driscoll S, Lawlor D (1999) Water stress inhibits plant photosynthesis by decreasing coupling factor and ATP. Nature 401:914–917
Tran T, Kano-Nakata M, Suralta R, Menge D, Mitsuya S, Inukai Y, Yamauchi A (2015) Root plasticity and its functional roles were triggered by water deficit but not by the resulting changes in the forms of soil N in rice. Plant Soil 386:65–76
Turner NC (1988) Measurement of plant water status by the pressure chamber technique. Irrig Sci 9:289–308
Vandeleur RK, Mayo G, Shelden MC, Gilliham M, Kaiser BN, Tyerman SD (2009) The role of plasma membrane intrinsic protein aquaporins in water transport through roots: diurnal and drought stress responses reveal different strategies between isohydric and anisohydric cultivars of grapevine. Plant Physiol 149:445–460
Wan X, Steudle E, Hartung W (2004) Gating of water channels (aquaporins) in cortical cells of young corn roots by mechanical stimuli (pressure pulses): effects of ABA and of HgCl2. J Exp Bot 55:411–422
Wang H, Liu F, Andersen MN, Jensen CR (2009) Comparative effects of partial root-zone drying and deficit irrigation on nitrogen uptake in potatoes (Solanum tuberosum L.). Irrig Sci 27:443–448
Wang X, Vignjevic M, Liu F, Jacobse S, Jiang D, Wollenweber B (2015) Drought priming at vegetative growth stages improves tolerance to drought and heat stresses occurring during grain filling in spring wheat. Plant Growth Regul 75(3):677–687
White PJ, George TS, Gregory PJ, Bengough AG, Hallett PD, McKenzie BM (2013) Matching roots to their environment. Ann Bot Lond 112:207–222
Wignarajah K (1990) Growth response of Phaseolus vulgaris to varying salinity regimes. Environ Exp Bot 30:141–147
Wilkinson S, Davies WJ (1997) Xylem sap pH increase: a drought signal received at the apoplastic face of the guard cell that involves the suppression of saturable abscisic acid uptake by the epidermal symplast. Plant Physiol 113:559–573
Yang Q, Zhang F, Li F, Liu X (2013) Hydraulic conductivity and water-use efficiency of young pear tree under alternate drip irrigation. Agric Water Manag 119:80–88
Zhou Q, Ravnskov S, Jiang D, Wollenweber B (2015) Changes in carbon and nitrogen allocation, growth and grain yield induced by arbuscular mycorrhizal fungi in wheat (Triticum aestivum L.) subjected to a period of water deficit. Plant Growth Regul 75(3):751–760
Acknowledgments
The authors are grateful for research Grants from the National Natural Science Foundation of China (51079124), the National High Technology Research and Development Program of China (2011AA100504),the Fundamental Research Funds for the Central Universities of China (QN2011067), 111 Project (No. B12007), and Technology Foundation for Selected Overseas Chinese Scholar of Shaanxi.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Niu, X., Hu, T., Zhang, F. et al. Severity and duration of osmotic stress on partial root system: effects on root hydraulic conductance and root growth. Plant Growth Regul 79, 177–186 (2016). https://doi.org/10.1007/s10725-015-0123-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-015-0123-1