Abstract
Although brassinosteroid (BR) has been suggested to play a role in strawberry fruit ripening, the defined function of this hormone remains unclear in the fruit. Here, BR content and BR receptor gene FaBRI1 expression were analysed during ‘Akihime’ strawberry fruit development. We found that BR levels increased during the later developmental stages, and the mRNA expression levels of FaBRI1 increased rapidly from white to initial red stages, suggesting that BR is associated with fruit ripening. This was further confirmed by exogenous application of BR and its inhibitor brassinazole (BZ) to big-green fruit, which significantly promoted and inhibited strawberry fruit ripening, respectively. More importantly, down-regulation of FaBRI1 expression in de-greening fruit markedly retarded strawberry red-colouring. In conclusion, we have provided physiological and molecular evidence to demonstrate that BR plays a role in strawberry fruit ripening. In addition, both BR content and FaBRI1 expression reached their peak levels in small-green fruit, suggesting that BR might also be involved in early strawberry fruit development. Further experiments are required to validate the role of BR in strawberry fruit cell division.
Similar content being viewed by others
Introduction
The phytohormone brassinosteroid plays an important role in various aspects of plant physiological responses including cell elongation and division, vascular differentiation, flowering, pollen development and photomorphogenesis (Clouse 2011a). Several reports have also shown that brassinosteroids are involved in fleshy fruit development and ripening of tomato fruit (Vidya Vardhini and Rao 2002; Lisso et al. 2006), grape berry (Symons et al. 2006) and cucumber (Fu et al. 2008). Although a previous report suggests that brassinosteroid might be involved in strawberry fruit ripening (Bombarely et al. 2010), its defined function remains unclear.
The ‘Brassins’ are the most active growth-promoting extracts isolated from Brassica napus pollen (Mitchell et al. 1970). Subsequently, another extract was identified as a steroidal lactone, and named brassinolide (BL) (Grove et al. 1979). Recently, many BL-like compounds, as animal hormone steroids, have been found throughout the plant kingdom and thus named brassinosteroids (BRs) collectively (Clouse and Sasse 1998). The genes that are involved in BR biosynthesis and action were identified mainly using a series of BR-deficient and BR-insensitive mutants in Arabidopsis (Yokota 1997; Clouse 2002; Fujioka and Yokota 2003; Vert et al. 2005; Bajguz 2007; Bishop 2007; Gendron and Wang 2007; Li and Jin 2007; Symons et al. 2008; Ohnishi et al. 2009; Kim and Wang 2010; Li 2010; Clouse 2011b). Plant sterols, which can be converted to BL via teasterone, typhasterol and castasterone, are synthesised by an isoprenoid biosynthetic pathway including acetyl-CoA, mevalonate, isopentenyl pyrophosphate, geranyl pyrophosphate and farnesyl pyrophosphate (Yokota 1997; Fujioka and Yokota 2003; Bajguz 2007; Bishop 2007; Symons et al. 2008; Ohnishi et al. 2009). BR perception and signalling transduction are involved in a series of reversible phosphorylation and dephosphorylation processes. The perception of BR by the BR receptor BRI1 at the cell surface leads to the dissociation of BRI1 kinase inhibitor BKI1 from the plasma membrane and association of BRI1 with its co-receptor BAK1. BRI1 phosphorylation of the BSK kinases activates the BSU1 phosphatases, which dephosphorylate and inactivate BIN2 kinase, resulting in the accumulation of unphosphorylated BES1/BZR1 transcription factors in the nucleus and subsequent activation of the downstream transcriptional network (Clouse 2002; Vert et al. 2005; Gendron and Wang 2007; Li and Jin 2007; Kim and Wang 2010; Li 2010; Clouse 2011b).
Following advances in model plant systems, Lisso et al. (2006) found that application of BRs to grape berries significantly promoted ripening, while brassinazole (an inhibitor of BR biosynthesis) significantly delayed fruit ripening. Grape BR receptor gene brassinosteroid insensitive 1 expression was consistent with the significant increase in endogenous BR levels observed at the onset of fruit ripening. Furthermore, a grape BR biosynthesis enzyme gene brassinosteroid-6-oxidase could rescue the tomato (Lycopersicon esculentum) extreme dwarf (dx/dx) mutant, demonstrating that BRs are involved in grape berry ripening (Symons et al. 2006). Similarly, application of BRs to tomato pericarp discs also elevated lycopene levels and lowered chlorophyll levels, indicating that BRs accelerate tomato fruit ripening and senescence (Vidya Vardhini and Rao 2002). A tomato d(x) mutant with severe symptoms of brassinosteroid-deficiency showed reduced dry mass content and levels of starch and various sugars, which can be rescued by BR application, demonstrating that brassinosteroid is required for tomato fruit development (Lisso et al. 2006). In cucumber, it is demonstrated that BRs play an important role during early fruit development (Fu et al. 2008). Recently, a collection of expressed sequence tags (ESTs) from cDNA libraries prepared from different strawberry fruit components and developmental stages showed that FaBRI1 receptor expression was increased in receptacles and clearly increased during ripening (Bombarely et al. 2010). However, to our knowledge, the substantial evidence for action of this hormone on strawberry fruit ripening has yet been lacking
In this study, BR levels and expression of the BR receptor gene FaBRI1 in ‘Akihime’ strawberry fruit suggested that BRs are involved in the ripening. This was further confirmed by application of BL or brassinazole (BZ) to strawberry fruit. Importantly, using the recently reported tobacco rattle virus-induced gene silencing (VIGS) in strawberry fruit (Jia et al. 2011; Chai et al. 2011), downregulation of FaBRI1 transcripts inhibited fruit red-colouring. In conclusion, these results provide physiological and molecular evidence to demonstrate that BR is involved in strawberry fruit ripening.
Materials and methods
Plant materials
Strawberry (Fragaria × ananassa Akihime) fruit were obtained from local producers (Beijing University of Agriculture, China). Fruits were harvested at different ripening stages and classified according to their external coloration. During strawberry development, fruits were collected on the following days (d) after anthesis: 7 d (small green), 14 d (big green), 16 d (de-greening), 21 d (white), 23 d (initial red), 25 d (partial red), and 27 d (full red). Ten fruits of uniform size were selected at each sampling time (one replication). In total, there were three replications of sampling times. Seeds from the receptacle were removed and cut into small cubes of 0.5–0.8 cm3, then quickly frozen in liquid nitrogen and stored at −80 °C for RNA extraction.
Effects of epibrassinolide and its inhibitor brassinazole on strawberry fruit ripening
To determine the relationship of BR with the onset of fruit ripening, 0.5 ml of 400 mM epibrassinolide (BL) or 200 mM its biosynthesis inhibitor brassinazole (BZ) were injected using a syringe into the receptacles of developmental fruit still attached to the plants during BG stage, respectively. Three continuous injections were performed once a day, and water was used as a control.
Cloning of the coding sequence of FaBRI1
Total RNA was isolated from strawberry fruit using an RNA Extraction Kit (Biomed Company, China). Genomic DNA was removed by 15-min incubation at 37 °C with RNase-Free DNase (TaKaRa, Otsu, Japan) followed by an RNA Clean Purification Kit (BioTeke, Beijing, China). The purity and integrity of RNA were analysed both by agarose gel electrophoresis and by the A260:A230 and A260:A280 ratios. To generate first strand cDNA, 3 μg of total RNA was reverse-transcribed using a SMART™ RACE cDNA Synthesis Kit (TaKaRa) according to the manufacturer’s protocol. The cDNA was used as a template for amplifying FaBRI1 with specific primers (forward, 5′-ATGAAACCCCACAGACCCGTTTCACC-3′; reverse, 5′-CTACTGCTTGCCCGGTTCAGGGTC-3′) designed from a strawberry gene library (https://strawberry.plantandfood.co.nz/index.html ). PCR was performed under the following conditions: 94 °C for 5 min, followed by 35 cycles of 94 °C for 30 s, 65 °C for 30 s, and 72 °C for 1 min, with the final reaction being terminated at 72 °C for an additional 10 min. The PCR products were ligated into a pUC-T vector and subsequently transformed into Escherichia coli DH5a. Positive colonies were selected, amplified, and then sequenced by Invitrogen China (Shanghai, China).
Viral vector constructs
The cDNA obtained above was used as a template to amplify FaBRI1 fragment with specific primers for viral vector constructs (forward, 5′-GCAGGGAGTG TGGCAATGGG-3′; reverse, 5′-GTGGTGAAGA AAGGCCAGTC-3′). PCR was performed under the following conditions: 94 °C for 5 min, followed by 35 cycles of 94 °C for 30 s, 53 °C for 30 s, and 72 °C for 1 min, with the final reaction being terminated at 72 °C for an additional 10 min. The PCR products were cloned using the pUC-T vector mentioned above. The fragment of FaBRI1 gene was then cut from the T-vector with Sac I and Xba I, and the fragment was ligated into the viral vector pTRV2 with Sac I and Xba I.
Agrobacterium-infiltration
VIGS assays were performed as described by Chai et al. (2011) and Liu et al. (2002) with minor modifications. Briefly, pTRV1, pTRV2, or the recombinant plasmid (pTRV2-FaBRI1) were transformed separately into Agrobacterium tumefaciens strain GV3101 using the freeze thaw method. A 5-ml culture of each transformant strain was grown overnight at 28 °C in LB medium containing 50 mg ml−1 kanamycin and 50 mg ml−1 rifampicin, 10 mM MES pH 5.6, and 20 mM acetosyringone. Each overnight culture was inoculated into 50 ml LB medium and again grown at 28 °C overnight. Cells were harvested by centrifugation at 5,000 × g for 5 min at 20 °C, then resuspended in infiltration buffer containing 10 mM MgCl2, 10 mM MES pH 5.6, and 200 mM acetosyringone, adjusted to a OD600 of 1.0–2.0, and shaken for 3 h at room temperature before infiltration. A 1:1 (v/v) ratio mixture containing pTRV1 plus pTRV2 or pTRV2-FaBRI1 was then infiltrated using an injector (5 ml) with a needle. The injection volume of the mixture was 0.2–0.4 ml per fruit.
Real-time RT-PCR
Real-time PCR reactions (20 μl) contained 10 μl SYBR Premix Ex Taq (perfect real-time buffer contained dNTPs, MgCl2, and DNA polymerase; TaKaRa), 0.4 μl 10 μM forward specific primer, 0.4 μl 10 μM reverse specific primer (Sangon, Shanghai, P. R. China), and 2 μl cDNA template mentioned above. The mixture was placed in a Bio-Rad iQ5 Sequence Detector (Bio-Rad, Hercules, CA, USA), and DNA amplification was conducted using the following thermocycling program: 1 cycle of 95 °C for 2 min, and 40 cycles of template denaturation at 94 °C for 20 s, primer annealing at 56 °C for 20 s, and primer extension at 72 °C for 30 s, and 71 cycles increasing from 60 to 95 °C at 0.5 °C per cycle for 30 s. The sequence detector was programmed to measure fluorescence only during the annealing step. At this temperature, no incorporated uniprimer was found in the hairpin conformation contributing to fluorescence measurements. The primers for real-time PCR were as follows:
FaBRI1: forward, 5′-AAACCTTGTTCCGCTCCT-3′; and reverse, 5′-CAATGGCAATCTCCCTCC-3′
Actin: forward 5′-GTATGGTCAAGGCTGGGTT-3′, and reverse 5′-CACGATTAGCCTTGGGATT-3′
Determination of BR content
For BR extraction, 0.5 g of receptacle was ground in a mortar and homogenised in extraction solution (isopropyl alcohol). Extracts were centrifuged at 10, 000 × g for 20 min. The suspension was collected and stored in the refrigerator at 4 °C for enzyme-linked immunosorbent assay (ELISA). ELISA procedures were conducted according to the manufacturer’s instructions (China Agricultural University, Beijing, China). BR analysis was measured using a Mul-tiskan MK3 microplate reader (Thermo Labsystems, USA).
Results
Effects of BL and BZ on strawberry fruit ripening
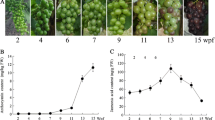
Following recent reports (Jia et al. 2011; Chai et al. 2011), seven developmental stages of ‘Akihime’ strawberry fruit have been established, including small green (SG), big green (BG), de-greening (DG), white (Wt), initial red (IR), partial red (PR) and full red (FR) respectively at 7, 14, 16, 21, 23, 25 and 27 days after anthesis under greenhouse conditions (Fig. 1).
Effects of epibrassinolide (BL) and its inhibitor brassinazole (BZ) on strawberry fruit ripening. Natural big fruit was treated with 400 mM BL and 200 mM BZ by application of 0.5 ml. Water treatment was used as the control. SG Small green, BG Big green, DG De-greening, Wt White, IR Initial red, PR Partial red, FR Full red
To explore the role of BR in strawberry fruit ripening, 0.5 ml of 400 mM epibrassinolide (BL) or 200 mM its biosynthesis inhibitor brassinazole (BZ) were injected with a syringe into the receptacles of developmental fruits still attached to the plant during the BG stage, respectively, while water was used as the control. The results showed that the application of BL or BZ could promote or inhibit strawberry fruit ripening ahead of 5 days or delay of 7 days compared with the control, respectively (Fig. 1). These results showed that BR could accelerate strawberry fruit ripening.
Cloning and characterisation of FaBRI1
To clone BRI1 homology gene from ‘Akihime’ strawberry fruit, an Arabidopsis BRI1 gene (At4g39400) sequence was used for BLAST analysis in a strawberry gene library (https://strawberry.plantandfood.co.nz/index.html) to identify homologs with gene loci 21119. Based on the nucleotide sequences, specific primers (FaBRI1: forward 5′-ATGAAACCCCACAGACCCGTTTCACC-3′ and reverse 5′-CTACTGCTTGCCCGGTTCAGGGTC-3′) were used to clone the FaBRI1coding sequence. Our results showed that 3,555-bp cDNA sequences that encoded the deduced 1184 amino acids of FaBRI1 was cloned (Fig. 2, GenBank submission ID: JX073557). The putative leucine-rich repeats (LRRs) and serine/threonine kinases domains in FaBRI1 (Fig. 3) were detected by homology analysis using the FaBRI1 protein on the NCBI website, http://blast.ncbi.nlm.nih.gov/Blast.cgi, suggesting that the putative FaBRI1 gene was isolated successfully from strawberry.
Putative conserved domains in FaBRI1. LRR-RI superfamily: Leucine-rich repeats (LRRs) and ribonuclease inhibitor (RI)-like subfamily. LRRs are 20–29 residue sequence motifs present in many proteins that participate in protein–protein interactions and have different functions and cellular locations. Protein Tyrosine Kinase (PTK) family catalytic domain: This PTKc family is part of a larger superfamily that includes the catalytic domains of protein serine/threonine kinases
Changes in BR content and FaBRI1 expression during strawberry fruit development
To further explore the role of BR in strawberry fruit ripening, the BR levels and FaBRI1 expression in seven-stage developmental fruit were analysed. The results showed that BR levels were extremely high in SG fruit, after which it decreased sharply and maintained stable and low levels until the onset of red-colour development. When the fruit underwent rapid red-colouring, this hormone significantly increased (Fig. 4). The mRNA expression levels of FaBRI1 were higher in SG fruit, but decreased rapidly up to the DG stage, followed by a rapid increase from Wt to IR stages (Fig. 4). These results suggested that BR might play a role in the ripening.
Downregulation of BR receptor FaBRI1 expression inhibits strawberry ripening
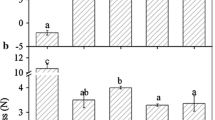
To further confirm the role of FaBRI1 in strawberry fruit development, we first cloned a 599-bp cDNA fragment of the FaBRI1 gene (from 2,362 to 2,961 bp) based on the strawberry FaBRI1 mRNA gene submitted to GenBank (Accession Number JX073557) from ‘Akihime’ fruit. Then, the cDNA sequence (named FaBRI1 599 ) was inserted into multiple cloning sites of the TRV2 virus vector with Sac I and Xba I. To silence the FaBRI1 gene in strawberry fruit, a mixture of Agrobacterium strain GV3101 cultures containing pTRV1 and pTRV2 or pTRV2-FaBRI1 599 at a 1:1 ratio was syringe-inoculated into de-greening fruit (Fig. 1). Control fruit was inoculated with TRV alone (empty vector). After 1.5 weeks, the surface of the control fruit turned fully red (Fig. 5a); in contrast, the inoculated sector on the surface of the RNAi fruit remained white (Fig. 5b). Real-time PCR analysis showed that FaBRI1 expression was significantly downregulated in the RNAi fruits compared to control fruit (Fig. 5c). These results indicated that FaBRI1 played an important role in strawberry fruit ripening.
Virus-induced FaBRI1 gene silencing in strawberry fruit. De-greening fruit still attached to the plant were inoculated with Agrobacterium-containing tobacco rattle virus (TRV) alone (control), or TRV carrying a FaBR1 fragment (RNAi). About 10 days after inoculation, the phenotypes of the control fruit (a) and RNAi fruit (b) were observed, respectively. Real-time PCR analyses of the FaBRI1 transcript levels in receptacles of the control or RNAi fruit (c) were conducted. Actin was used as the internal control. Error bars represent the SE (n = 3)
Discussion
In recent years, loss-of-function mutants deficient in BR biosynthesis or perception with various phenotypes including dwarf plant, dark-green and epinastic leaves, reduced apical dominance, delayed flowering and senescence, altered vascular patterning, and male sterility, have been extensively studied in Arabidopsis (Clouse and Sasse 1998; Yokota 1997; Kim and Wang 2010; Clouse et al. 1996; Li et al. 1996; Sun et al. 2010). However, the role of BRs in fleshy fruit development and ripening has been less reported previously (Vidya Vardhini and Rao 2002; Lisso et al. 2006; Symons et al. 2006; Fu et al. 2008). Thus, more defined data on the role of BR in fleshy fruit are required to be given. Although a recent study suggests that BR might be involved in strawberry fruit ripening using the data gained from a collection of ESTs, to our knowledge, the role of this hormone in the fruit remains unclear.
In the present study, we provide physiological and molecular evidence to demonstrate that BR indeed plays a role in strawberry fruit ripening. We found that (1) BR levels increased during later fruit development, (2) exogenous application of BR and BZ significantly promoted and inhibited strawberry fruit ripening, respectively, (3) the mRNA expression levels of FaBRI1 increased rapidly from white to initial red stages, and (4) downregulation of FaBRI1 expression markedly retarded strawberry fruit red-colouring. These data demonstrate that BR is required for strawberry fruit ripening.
In addition, BR content and FaBRI1 expression peaked during the small-green stage. Given that it is also found that BR plays an important role in early cucumber fruit development (Fu et al. 2008), although the current study does not provide substantive evidence in this context, we also think that BR may play a role in early strawberry fruit development as the same as its role in fruit ripening. Further experiments are required to validate the role of BR in strawberry fruit cell division.
Conclusions
In the present study, we demonstrated that BR plays an important role in strawberry fruit ripening, and may be involved in early fruit development. The supported data is gained from the changes in BR content and FaBRI1 expression in developmental fruit, as well as the effects of exogenous application of BR and BZ on ripening. The most important evidence is gained from FaBRI1 RNAi tests by VIGS (downregulation of FaBRI1 expression), which markedly retarded strawberry red-colouring.
Abbreviations
- BR:
-
Brassinosteroid
- BZ:
-
BR inhibitor brassinazole
- FaBRI1:
-
Strawberry brassinosteroid receptor
- VIGS:
-
Virus-induced gene silencing
- LRR:
-
Leucine-rich repeat
- BKI1:
-
BRI1 kinase inhibitor 1
- BSK1:
-
BR signalling kinase 1
- BIN2:
-
Brassinosteroid insensitive 2
- BZR1:
-
Brassinazole resistant 1
References
Bajguz A (2007) Metabolism of brassinosteroids in plants. Plant Physiol Biochem 45:95–107
Bishop GJ (2007) Refining the plant steroid hormone biosynthesis pathway. Trends Plant Sci 12:377–380
Bombarely A, Merchante C, Csukasi F, Cruz-Rus E, Caballero JL, Medina-Escobar N, Blanco-Portales R, Botella MA, Muñoz-Blanco J, Sánchez-Sevilla JF, Valpuesta V (2010) Generation and analysis of ESTs from strawberry (Fragaria × ananassa) fruits and evaluation of their utility in genetic and molecular studies. BMC Genomics 11:503
Chai YM, Jia HF, Li CL, Dong QH, Shen YY (2011) FaPYR1 is involved in strawberry fruit ripening. J Exp Bot 62:5079–5089
Clouse SD (2002) Brassinosteroid signal transduction: clarifying the pathway from ligand perception to gene expression. Mol Cell 10:973–982
Clouse SD (2011a) Brassinosteroids. Arabidopsis Book 9: e0151
Clouse SD (2011b) Brassinosteroid signal transduction: from receptor kinase activation to transcriptional networks regulating plant development. Plant Cell 23:1219–1230
Clouse SD, Sasse JM (1998) Brassinosteroids: essential regulators of plant growth and development. Annu Rev Plant Physiol Plant Mol Biol 49:427–451
Clouse SD, Langford M, McMorris TC (1996) A brassinosteroid insensitive mutant in Arabidopsis thaliana exhibits multiple defects in growth and development. Plant Physiol 111:671–678
Fu FQ, Mao WH, Shi K, Zhou YH, Asami T, Yu JQ (2008) A role of brassinosteroids in early fruit development in cucumber. J Exp Bot 59:2299–2308
Fujioka S, Yokota T (2003) Biosynthesis and metabolism of brassinosteroids. Ann Rev Plant Biol 54:137–164
Gendron JM, Wang ZY (2007) Multiple mechanisms modulate brassinosteroid signaling. Curr Opin Plant Biol 10:436–441
Grove MD, Spencer GF, Rohwedder WK, Mandava NB, Worley JF, Warthen JD, Steffens GL, Flippen-Anderson JL, Cook JC (1979) Brassinolide, a plant growth-promoting steroid isolated from Brassica napus pollen. Nature 281:216–217
Jia HF, Chai YM, Li CL, Lu D, Luo JJ, Qin L, Shen YY (2011) Abscisic acid plays an important role in the regulation of strawberry fruit ripening. Plant Physiol 157:188–199
Kim TW, Wang ZY (2010) Brassinosteroid signal transduction from receptor kinases to transcription factors. Annu Rev Plant Biol 61:681–704
Li J (2010) Regulation of the nuclear activities of brassinosteroid signaling. Curr Opin Plant Biol 13:540–547
Li J, Jin H (2007) Regulation of brassinosteroid signaling. Trends Plant Sci 12:37–41
Li J, Nagpal P, Vitart V, McMorris TC, Chory J (1996) A role for brassinosteroids in light-dependent development of Arabidopsis. Science 272:398–401
Lisso J, Altmann T, Müssig C (2006) Metabolic changes in fruits of the tomato dx mutant. Phytochemistry 67:2232–2238
Liu Y, Schiff M, Dinesh-Kumar SP (2002) Virus-induced gene silencing in tomato. Plant J 31:777–786
Mitchell JW, Mandava NB, Worley JF, Plimmer JR, Smith MV (1970) Brassins: a new family of plant hormones from rape pollen. Nature 225:1065–1066
Ohnishi T, Yokota T, Mizutani M (2009) Insights into the function and evolution of P450 s in plant steroid metabolism. Phytochemistry 70:1918–1929
Sun Y, Fan XY, Cao DM, Tang W, He K, Zhu JY, He JX, Bai MY, Zhu S, Oh E, Patil S, Kim TW, Ji H, Wong WH, Rhee SY, Wang ZY (2010) Integration of brassinosteroid signal transduction with the transcription network for plant growth regulation in Arabidopsis. Dev Cell 19:765–777
Symons GM, Davies C, Shavrukov Y, Dry IB, Reid JB, Thomas MR (2006) Grapes on steroids: brassinosteroids are involved in grape berry ripening. Plant Physiol 140:150–158
Symons GM, Ross JJ, Jager CE, Reid JB (2008) Brassinosteroid transport. J Exp Bot 59:17–24
Vert G, Nemhauser JL, Geldner N, Hong F, Chory J (2005) Molecular mechanisms of steroid hormone signaling in plants. Ann Rev Cell Dev Biol 21:177–201
Vidya Vardhini B, Rao SS (2002) Acceleration of ripening of tomato pericarp discs by brassinosteroids. Phytochemistry 61:843–847
Yokota T (1997) The structure, biosynthesis and function of brassinosteroids. Trends Plant Sci 2:137–143
Acknowledgments
We would like to thank Prof. Liu Yu-le (Qinghua University, Beijing China) for pTRV vectors. This work was supported financially by the National Basic Research “973” Program of China (grant no. 2012CB126306), the China National Natural Science Foundation (grant no. 30971977, 31272144), and the Newstar of Science and Technology Supported by Beijing Metropolis (grant no. Z111105054511048).
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ye-mao Chai, Qing Zhang and Lin Tian authors contributed equally to this work.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Chai, Ym., Zhang, Q., Tian, L. et al. Brassinosteroid is involved in strawberry fruit ripening. Plant Growth Regul 69, 63–69 (2013). https://doi.org/10.1007/s10725-012-9747-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-012-9747-6