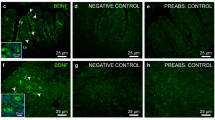
Abstract
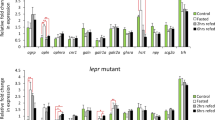
The biological actions of growth hormone (GH) are pleiotropic, including growth promotion, energy mobilization, gonadal development, appetite, and social behavior. The regulatory network for GH is complex and includes many central and peripheral endocrine factors as well as that from the environment. It is known that GH transgenesis results in increased growth, food intake, and consequent metabolic rates in fishes. However, the manner in which GH transgenesis alters the energetic metabolism in fishes has not been well explored. In order to elucidate these consequences, we examined the effect of GH overexpression on appetite control mechanisms in a transgenic zebrafish (Danio rerio) model. To this, we analyzed feeding behavior and the expression of the main appetite-related genes in two different feeding periods (fed and fasting) in non-transgenic (NT) and transgenic (T) zebrafish as well as glycaemic parameters of them. Our initial results have shown that NT males and females present the same feeding behavior and expression of main appetite-controlling genes; therefore, the data of both sexes were properly grouped. Following grouped data analyses, we compared the same parameters in NT and T animals. Feeding behavior results have shown that T animals eat significantly more and faster than NT siblings. Gene expression results pointed out that gastrointestinal (GT) cholecystokinin has a substantial contribution to the communication between peripheral and central control of food intake. Brain genes expression analyses revealed that T animals have a down-regulation of two strong and opposite peptides related to food intake: the anorexigenic proopiomelanocortin (pomc) and the orexigenic neuropeptide Y (npy). The down-regulation of pomc in T when compared with NT is an expected result, since the decrease in an anorexigenic factor might keep the transgenic fish hungry. The down-regulation of npy seemed to be contradictory at first, but if we consider the GH’s capacity to elevate blood glucose, and that NPY is able to respond to humoral factors like glucose, this down-regulation makes sense. In fact, our last experiment showed that transgenics presented elevated blood glucose levels, confirming that npy might responded to this humoral factor. In conclusion, we have shown that GT responds to feeding status without interference of transgenesis, whereas brain responds to GH transgenesis without any effect of treatment. It is clear that transgenic zebrafish eat more and faster, and it seems that it occurs due to pomc down-regulation, since npy might be under regulation of the humoral factor glucose.


Similar content being viewed by others
References
Abrahams M, Sutterlin A (1999) The foraging and antipredator behaviour of growth-enhanced transgenic Atlantic salmon. Anim Behav 58:933–942
Alrubaian J, Sollars C, Danielson PB, Dores RM (2003) Evaluating the radiation of the POMC gene in teleosts: characterization of American eel POMC. Gen Comp Endocrinol 132:384–390
Burdakov D, Gerasimenko O, Verkhratsky A (2005a) Physiological changes in glucose differentially modulate the excitability of hypothalamic melanin-concentrating hormone and orexin neurons in situ. J Neurosci 25:2429–2433
Burdakov D, Luckman SM, Verkhratsky A (2005b) Glucose-sensing neurons of the hypothalamus. Philos Trans R Soc B 360:2227–2235
Campfield LA, Smith FJ, Guisez Y et al (1995) Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science 269:546–549
Canosa LF, Unniappan S, Peter RE (2005) Periprandial changes in growth hormone release in goldfish: role of somatostatin, ghrelin, and gastrin-releasing peptide. Am J Physiol Reg I 289:R125–R133
Cerdá-Reverter JM (2003) Molecular cloning, pharmacological characterization, and brain mapping of the melanocortin 4 receptor in the goldfish: involvement in the control of food intake. Endocrinology 144:2336–2349
Chan YY, Steiner RA, Clifton DK (1996) Regulation of hypothalamic neuropeptide-Y neurons by growth hormone in the rat. Endocrinology 137:1319–1325
Chiu CN, Prober DA (2013) Regulation of zebrafish sleep and arousal states: current and prospective approaches. Front Neural Circuits 7:58
Company R, Astola A, Pendón C et al (2001) Somatotropic regulation of fish growth and adiposity: growth hormone (GH) and somatolactin (SL) relationship. Comp Biochem Phys C 130:435–445
Devlin RH, Johnsson JI, Smailus DE et al (1999) Increased ability to compete for food by growth hormone-transgenic coho salmon Oncorhynchus kisutch (Walbaum). Aquac Res 30:479–482
Dores RM, Cameron E, Lecaude S, Danielson PB (2003) Presence of the δ-MSH sequence in a proopiomelanocortin cDNA cloned from the pituitary of the galeoid shark, Heterodontus portusjacksoni. Gen Comp Endocrinol 133:71–79
Facciolo RM, Crudo M, Giusi G et al (2009) Light- and dark-dependent orexinergic neuronal signals promote neurodegenerative phenomena accounting for distinct behavioral responses in the teleost Thalassoma pavo. J Neurosci Res 87:748–757
Figueiredo MA, Lanes CFC, Almeida DV, Marins LF (2007) Improving the production of transgenic fish germlines: in vivo evaluation of mosaicism in zebrafish (Danio rerio) using a green fluorescent protein (GFP) and growth hormone cDNA transgene co-injection strategy. Genet Mol Biol 30:31–36
Gonzalez R, Unniappan S (2010) Molecular characterization, appetite regulatory effects and feeding related changes of peptide YY in goldfish. Gen Comp Endocrinol 166:273–279
Halaas J, Gajiwala K, Maffei M et al (1995) Weight-reducing effects of the plasma protein encoded by obese gene. Science 269:543–546
Halford JCG, Cooper GD, Dovey TM (2004) The pharmacology of human appetite expression. Curr Drug Targets 5:221–240
Hallerman EM, McLean E, Fleming IA (2007) Effects of growth hormone transgenes on the behavior and welfare of aquacultured fishes: a review identifying research needs. Appl Anim Behav Sci 104:265–294
Hemre G, Mommsen TP, Krigdahl A (2002) Carbohydrates in fish nutrition effects on growth, glucose metabolism and hepatic enzymes. Aquac Nutr 8:175–194
Himick BA, Peter RE (1994) Bombesin acts to suppress feeding behavior and alter serum growth hormone in goldfish. Physiol Behav 55:65–72
Holt RIG, Simpson HL, Sönksen PH (2003) The role of the growth hormone-insulin-like growth factor axis in glucose homeostasis. Diabet Med 20:3–15
Hoskins LJ, Volkoff H (2012) The comparative endocrinology of feeding in fish: insights and challenges. Gen Comp Endocrinol 176:327–335
Johnsson JI, Bjornsson BT (1994) Growth hormone increases growth rate, appetite and dominance in juvenile rainbow trout, Oncorhynchus mykiss. Anim Behav 48:177–186
Jonsson E, Johnsson JI, Bjornsson BT (1996) Growth hormone increase predation exposure of rainbow trout. Proc R Soc B 263:647–651
Jönsson E, Johnsson JI, Björnsson BT (1998) Growth hormone increases risk-taking in foraging rainbow trout. Ann NY Acad SCI 839:636–638
Knable AE (1973) A comparison of the food intake of male and female channel catfish (Ictalurus punctatus). Trans Illinois Acad Sci 66:41–43
Koven W, Schulte P (2012) The effect of fasting and refeeding on mRNA expression of PepT1 and gastrointestinal hormones regulating digestion and food intake in zebrafish (Danio rerio). Fish Physiol Biochem 38:1565–1575
Kulczykowska E, Vázquez FJS (2010) Neurohormonal regulation of feed intake and response to nutrients in fish: aspects of feeding rhythm and stress. Aquac Res 41:654–667
Kuz’mina VV (2005) Regulation of the fish alimentary behavior: role of humoral component. J Evol Biochem Physiol 41:282–295
Le Bail P, Boeuf G (1997) What hormones may regulate food intake in fish? Aquat Living Resour 10:371–379
Leibowitz SF (1991) Brain neuropeptide Y: an integrator of endocrine, metabolic and behavioral processes. Brain Res Bull 27:333–337
Lin X, Volkoff H, Narnaware Y et al (2000) Brain regulation of feeding behavior and food intake in fish. Comp Biochem Phys A 126:415–434
Lõhmus M, Raven PA, Sundström LF, Devlin RH (2008) Disruption of seasonality in growth hormone-transgenic coho salmon (Oncorhynchus kisutch) and the role of cholecystokinin in seasonal feeding behavior. Horm Behav 54:506–513
MacDonald E, Volkoff H (2009a) Neuropeptide Y (NPY), cocaine- and amphetamine-regulated transcript (CART) and cholecystokinin (CCK) in winter skate (Raja ocellata): cDNA cloning, tissue distribution and mRNA expression responses to fasting. Gen Comp Endocrinol 161:252–261
MacDonald E, Volkoff H (2009b) Cloning, distribution and effects of season and nutritional status on the expression of neuropeptide Y (NPY), cocaine and amphetamine regulated transcript (CART) and cholecystokinin (CCK) in winter flounder (Pseudopleuronectes americanus). Horm Behav 56:58–65
Mauras N, Haymond MW (2005) Are the metabolic effects of GH and IGF-I separable? Growth Horm Igf Res 15:19–27
Merali Z, McIntosh J, Anisman H (1999) Role of bombesin-related peptides in the control of food intake. Neuropeptides 33:376–386
Metz JR, Peters JJM, Flik G (2006) Molecular biology and physiology of the melanocortin system in fish: a review. Gen Comp Endocrinol 148:150–162
Miquet JG, Sotelo AI, Bartke A, Turyn D (2004) Suppression of growth hormone (GH) Janus tyrosine kinase 2/signal transducer and activator of transcription 5 signaling pathway in transgenic mice overexpressing bovine GH. Endocrinology 145:2824–2832
Møller N, Nørrelund H (2003) The role of growth hormone in the regulation of protein metabolism with particular reference to conditions of fasting. Horm Res 59(1):62–68
Murashita K, Fukada H, Hosokawa H, Masumoto T (2006) Cholecystokinin and peptide Y in yellowtail (Seriola quinqueradiata): molecular cloning, real-time quantitative RT-PCR, and response to feeding and fasting. Gen Comp Endocrinol 145:287–297
Murashita K, Kurokawa T, Nilsen TO, Rønnestad I (2009) Ghrelin, cholecystokinin, and peptide YY in Atlantic salmon (Salmo salar): molecular cloning and tissue expression. Gen Comp Endocrinol 160:223–235
Nakamachi T, Matsuda K, Maruyama K et al (2006) Regulation by orexin of feeding behaviour and locomotor activity in the goldfish. J Neuroendocr 18:290–297
Panserat S, Kamalam BS, Fournier J et al (2014) Glucose metabolic gene expression in growth hormone transgenic coho salmon. Comp Biochem Phys A 170:38–45
Peng C, Humphries S, Peter RE et al (1993) Actions of goldfish neuropeptide Y on the secretion of growth hormone and gonadotropin-II in female goldfish. Gen Comp Endocrinol 90:306–317
Peyon P, Saied H, Lin X, Peter RE (1999) Postprandial, seasonal and sexual variations in cholecystokinin gene expression in goldfish brain. Mol Brain Res 74:190–196
Piccinetti CC, Migliarini B, Olivotto I et al (2010) Appetite regulation: the central role of melatonin in Danio rerio. Horm Behav 58:780–785. doi:10.1016/j.yhbeh.2010.07.013
Raven PA, Uh M, Sakhrani D et al (2008) Endocrine effects of growth hormone overexpression in transgenic coho salmon. Gen Comp Endocrinol 159:26–37
Rousseau K, Dufour S (2007) Comparative aspects of GH and metabolic regulation in lower vertebrates. Neuroendocrinology 86:165–174
Schwartz MW, Seeley RJ (1997) Neuroendocrine responses to starvation and weight loss. N Engl J Med 19:1802–1811
Silverstein JT, Wolters WR, Shimizu M, Dickhoff WW (2000) Bovine growth hormone treatment of channel catfish: strain and temperature effects on growth, plasma IGF-I levels, feed intake and efficiency and body composition. Aquaculture 190:77–88
Straus DS (1994) Nutritional regulation of hormones and growth factors that control mammalian growth. FASEB 8:6–12
Sundström LF, Devlin RH, Johnsson JI, Biagi CA (2003) Vertical position reflects increased feeding motivation in growth hormone transgenic coho salmon (Oncorhynchus kisutch). Ethology 109:701–712
Thorndyke MC, Reeve JR, Vigna SR (1990) Biological activity of a bombesin-like peptide extracted from the intestine of the ratfish, Hydrolagus colliei. Comp Biochem Phys C 96:135–140
Toguyeni A, Fauconneau B, Boujard T et al (1997) Feeding behaviour and food utilisation in tilapia, Oreochromis niloticus: effect of sex ratio and relationship with the endocrine status. Physiol Behav 62:273–279
Tsujino N, Sakurai T (2009) Orexin/hypocretin: a neuropeptide at the interface of sleep, energy homeostasis, and reward system. Pharmacol Rev 61:162–176
Valassi E, Scacchi M, Cavagnini F (2008) Neuroendocrine control of food intake. Nutr Metab Cardiovasc 18:158–168
Valen R, Jordal A, Murashita K, Rønnestad I (2011) Postprandial effects on appetite-related neuropeptide expression in the brain of Atlantic salmon, Salmo salar. Gen Comp Endocrinol 171:359–366
Vandesompele J, Preter KD, Pattyn F et al (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:1–12
Volkoff H (2006) The role of neuropeptide Y, orexins, cocaine and amphetamine-related transcript, cholecystokinin, amylin and leptin in the regulation of feeding in fish. Comp Biochem Phys A 144:325–331
Volkoff H, Peter RE (2006) Feeding behavior of fish and Its control. Zebrafish 3:131–140
Volkoff H, Canosa LF, Unniappan S et al (2005) Neuropeptides and the control of food intake in fish. Gen Comp Endocrinol 142:3–19
Volkoff H, Xu M, MacDonald E, Hoskins L (2009) Aspects of the hormonal regulation of appetite in fish with emphasis on goldfish, Atlantic cod and winter flounder: notes on actions and responses to nutritional, environmental and reproductive changes. Comp Biochem Phys A 153:8–12
Wall A, Volkoff H (2013) Effects of fasting and feeding on the brain mRNA expressions of orexin, tyrosine hydroxylase (TH), PYY and CCK in the Mexican blind cavefish (Astyanax fasciatus mexicanus). Gen Comp Endocrinol 183:44–52
Wang N, Hayward RS, Noltie DB (1998) Variation in food consumption, growth, and growth efficiency among juvenile hybrid sunfish held individually. Aquaculture 167:43–52
Yamanaka A, Muraki Y, Ichiki K et al (2006) Orexin neurons are directly and indirectly regulated by catecholamines in a complex manner. J Neurophysiol 96:284–298
Yousefian M, Shirzad E (2011) The review of the effect of growth hormone on immune system, metabolism and osmoregulation of fish. Aust J Basic Appl Sci 5:467–475
Zhdanova IV (2011) Sleep and its regulation in zebrafish. Rev Neurosci 22:27–36
Acknowledgments
This work was supported by Brazilian CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior). L. F. Marins is a research fellow from CNPq (Proc. No. 304675/2011-3).
Conflict of interest
Authors declare no conflict of interest.
Ethical standard
The experimental protocols used for animals in this study were conducted in accordance with Brazilian legislation on ethics in animal use.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dalmolin, C., Almeida, D.V., Figueiredo, M.A. et al. Food intake and appetite control in a GH-transgenic zebrafish. Fish Physiol Biochem 41, 1131–1141 (2015). https://doi.org/10.1007/s10695-015-0074-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-015-0074-5